Ch3 Ch2 Ch Ch3 2
thesills
Sep 13, 2025 · 6 min read
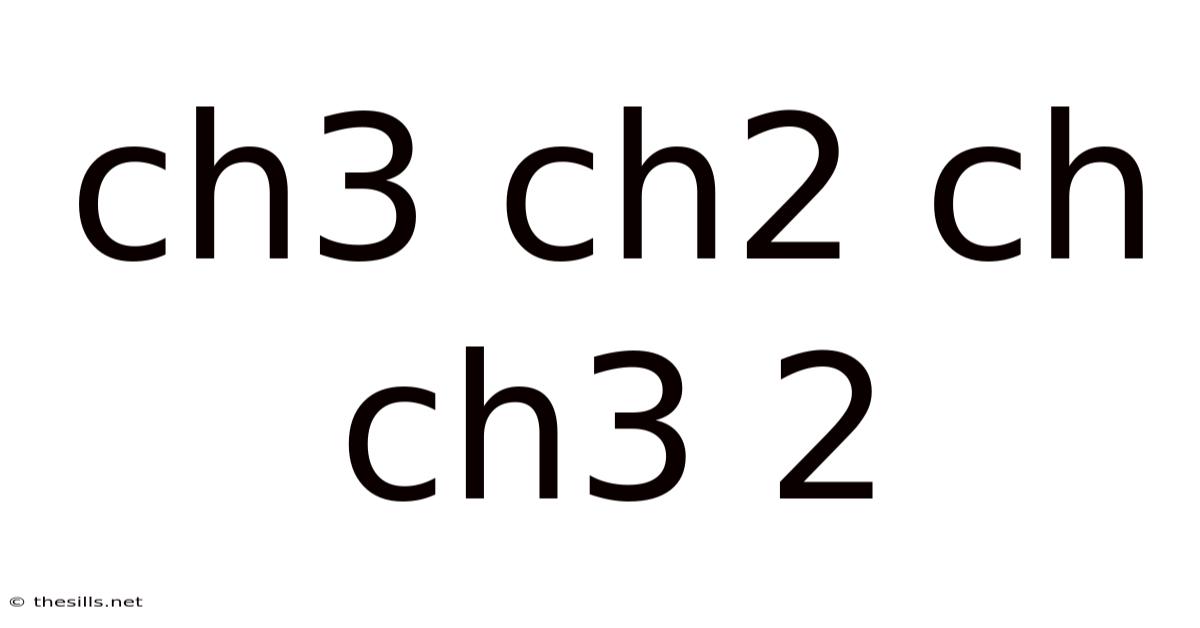
Table of Contents
Understanding the Structure and Properties of 2-Methylpentane (CH3CH2CH(CH3)CH2CH3)
This article delves into the fascinating world of organic chemistry, specifically focusing on the hydrocarbon 2-methylpentane, also represented by the structural formula CH3CH2CH(CH3)CH2CH3. We'll explore its structural characteristics, isomerism, physical properties, chemical properties, and potential applications. Understanding the properties of 2-methylpentane is crucial in various fields, from fuel science to organic synthesis.
Introduction to Alkanes and Isomerism
Before diving into the specifics of 2-methylpentane, let's establish a foundational understanding of alkanes and isomerism. Alkanes are saturated hydrocarbons, meaning they consist solely of carbon and hydrogen atoms, with single bonds connecting them. They follow the general formula C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms.
Isomerism is a crucial concept in organic chemistry. Isomers are molecules that share the same molecular formula but differ in their structural arrangement. 2-methylpentane is an example of a structural isomer. Structural isomers have the same atoms but arranged differently in the molecule's structure. This difference in arrangement significantly impacts the physical and chemical properties of the isomers.
For example, 2-methylpentane is an isomer of n-hexane (CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>). Both have the molecular formula C<sub>6</sub>H<sub>14</sub>, but their carbon chains are arranged differently, leading to variations in their boiling points, melting points, and reactivity.
The Structure of 2-Methylpentane
The structural formula CH3CH2CH(CH3)CH2CH3 clearly illustrates the arrangement of atoms in 2-methylpentane. It's a branched-chain alkane with six carbon atoms. A methyl group (CH<sub>3</sub>) is attached to the second carbon atom in the pentane chain. This branching significantly impacts its properties compared to its linear isomer, n-hexane.
-
Carbon Skeleton: The backbone of 2-methylpentane is a five-carbon chain (pentane), with a methyl group as a branch.
-
Bonding: All carbon-carbon and carbon-hydrogen bonds are single (sigma) bonds. This saturation contributes to its relatively lower reactivity compared to unsaturated hydrocarbons like alkenes or alkynes.
-
Conformations: Like all alkanes, 2-methylpentane exists in various conformations due to the rotation around its carbon-carbon single bonds. These conformations differ in energy, with some being more stable than others. However, these conformational changes do not alter its chemical identity.
Physical Properties of 2-Methylpentane
The physical properties of 2-methylpentane are largely determined by its molecular structure and intermolecular forces.
-
State at Room Temperature: 2-methylpentane is a colorless liquid at room temperature and standard atmospheric pressure.
-
Boiling Point: The boiling point of 2-methylpentane is lower than that of n-hexane. This is because branched-chain alkanes experience weaker intermolecular van der Waals forces compared to their linear counterparts. The more compact structure of 2-methylpentane reduces the surface area available for these interactions, resulting in a lower boiling point.
-
Melting Point: Similar to the boiling point, the melting point of 2-methylpentane is also lower than that of n-hexane. This is again due to the reduced intermolecular forces in the branched structure.
-
Density: 2-methylpentane has a lower density than water, meaning it is less dense and will float on water.
-
Solubility: Like other alkanes, 2-methylpentane is nonpolar and therefore insoluble in water (a polar solvent). It is, however, soluble in other nonpolar solvents such as other alkanes, ethers, and aromatic hydrocarbons.
-
Flammability: 2-methylpentane is a highly flammable liquid and should be handled with extreme caution away from open flames or ignition sources.
Chemical Properties of 2-Methylpentane
The chemical reactivity of 2-methylpentane is typical of alkanes. They are relatively unreactive due to the strong C-C and C-H single bonds.
-
Combustion: The primary chemical reaction of 2-methylpentane is combustion. When reacted with sufficient oxygen, it undergoes complete combustion to produce carbon dioxide and water:
2C<sub>6</sub>H<sub>14</sub> + 19O<sub>2</sub> → 12CO<sub>2</sub> + 14H<sub>2</sub>O
-
Halogenation: Under specific conditions (UV light or high temperature), 2-methylpentane can undergo halogenation reactions with halogens like chlorine or bromine. This substitution reaction replaces one or more hydrogen atoms with halogen atoms, forming haloalkanes. The reaction is free-radical in nature.
-
Isomerization: While not a common reaction under normal conditions, 2-methylpentane can be isomerized to its other isomers, particularly under the influence of catalysts.
-
Cracking: At high temperatures and pressures, in the presence of a catalyst, 2-methylpentane can undergo cracking, breaking down into smaller alkanes and alkenes. This is a crucial process in petroleum refining.
Applications of 2-Methylpentane
The relatively low reactivity and specific physical properties of 2-methylpentane make it useful in several applications:
-
Solvent: Its nonpolar nature makes it a useful solvent in certain industrial applications, especially in the dissolution of nonpolar substances.
-
Fuel Component: Due to its high flammability and energy content, 2-methylpentane is a component of gasoline and other fuels. The octane rating of gasoline is influenced by the presence of branched-chain alkanes like 2-methylpentane.
-
Organic Synthesis: It can serve as a starting material in the synthesis of other organic compounds, although it's not a widely used starting material due to its relative inertness. It might be used as a solvent in these syntheses.
-
Calibration Standard: Its well-defined properties make it suitable for use as a calibration standard in various analytical instruments.
Spectroscopic Characterization of 2-Methylpentane
Modern techniques like nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy are invaluable in confirming the identity and purity of 2-methylpentane.
-
NMR Spectroscopy: <sup>1</sup>H NMR spectroscopy would reveal distinct signals corresponding to the different types of protons in the molecule. The integration of these signals would reflect the relative number of each type of proton. <sup>13</sup>C NMR spectroscopy would provide information about the different carbon environments in the molecule.
-
IR Spectroscopy: IR spectroscopy would reveal characteristic absorption bands corresponding to C-H stretching and bending vibrations, as well as C-C stretching vibrations.
Frequently Asked Questions (FAQs)
-
Q: What is the difference between 2-methylpentane and n-hexane?
A: Both have the same molecular formula (C<sub>6</sub>H<sub>14</sub>), but 2-methylpentane is a branched-chain isomer of n-hexane, which is a linear chain. This difference in structure leads to variations in their physical properties (boiling point, melting point, etc.) and potentially some minor differences in chemical reactivity.
-
Q: Is 2-methylpentane toxic?
A: While not acutely toxic, prolonged exposure to 2-methylpentane vapors can cause irritation to the eyes, skin, and respiratory system. Proper ventilation and safety precautions should always be followed when handling this compound.
-
Q: What is the octane rating of 2-methylpentane?
A: The exact octane rating varies depending on the specific testing method, but it contributes positively to the octane rating of gasoline blends. Branched-chain alkanes generally have higher octane ratings compared to linear alkanes.
Conclusion
2-methylpentane, with its seemingly simple molecular structure, exhibits a fascinating array of properties influenced by its branched-chain arrangement. Its relatively low reactivity, specific physical properties, and role as a fuel component make it a significant compound in various industrial applications. Understanding its structure, properties, and reactivity is crucial for those working in the fields of chemistry, fuel science, and organic synthesis. Further research continues to explore its potential uses and interactions within complex systems. The information presented in this article provides a comprehensive overview of this fundamental organic molecule. Remember to always prioritize safety when handling any chemical substance.
Latest Posts
Latest Posts
-
Putting Food Into Your Mouth
Sep 13, 2025
-
X 2 3x 24 0
Sep 13, 2025
-
What Is 4 25 Of 10000
Sep 13, 2025
-
Are Anions Or Cations Bigger
Sep 13, 2025
-
3x 2 2x 5 Factored
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about Ch3 Ch2 Ch Ch3 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.