Balance Na H2o Naoh H2
thesills
Sep 12, 2025 · 6 min read
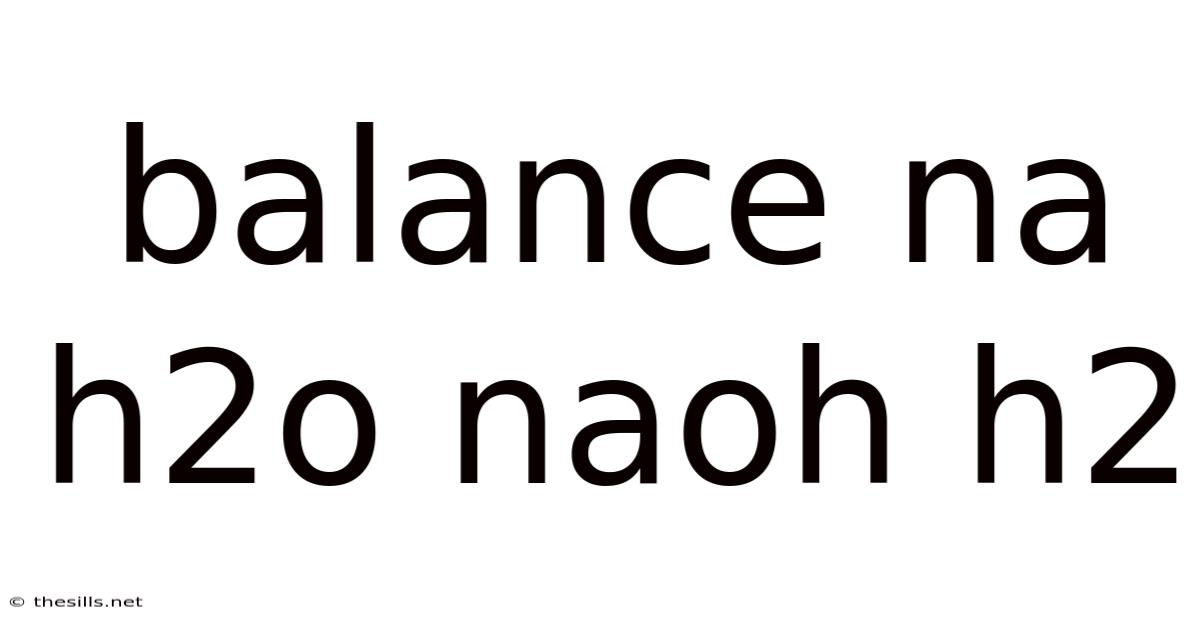
Table of Contents
Understanding the Balance: H₂O, NaOH, and H₂
The interaction between water (H₂O), sodium hydroxide (NaOH), and hydrogen gas (H₂) is a fundamental concept in chemistry, illustrating principles of acid-base reactions, redox reactions, and equilibrium. This article delves into the intricacies of these interactions, explaining the underlying chemistry in a clear and accessible manner. We will explore how these three substances relate, focusing on the reactions that occur and the factors influencing the balance between them. Understanding this balance is crucial in various applications, from industrial processes to everyday chemical phenomena.
Introduction: A Trio of Chemical Players
Water (H₂O), a ubiquitous solvent and essential for life, acts as the backdrop for many chemical reactions. Sodium hydroxide (NaOH), a strong alkali commonly known as lye or caustic soda, readily dissociates in water to produce hydroxide ions (OH⁻) and sodium ions (Na⁺). Hydrogen gas (H₂), a highly flammable and reactive diatomic molecule, is often produced through reactions involving acids or bases. The relationship between these three compounds is multifaceted and often involves redox reactions (reduction-oxidation), where electron transfer occurs.
The Reaction of Sodium Hydroxide with Water: Dissociation and Ionization
When sodium hydroxide (NaOH) is dissolved in water (H₂O), it undergoes complete dissociation. This means it separates completely into its constituent ions:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
This process releases a significant amount of heat, making the solution exothermic. The hydroxide ions (OH⁻) are responsible for the alkaline nature of the solution. The concentration of hydroxide ions directly determines the pH of the solution; a higher concentration signifies a higher pH (more alkaline). This dissociation is a crucial aspect of understanding how NaOH interacts with other substances, including hydrogen gas, indirectly. The presence of abundant hydroxide ions significantly influences the overall reaction environment.
The Role of Hydrogen Gas: Redox Reactions and its Limited Direct Interaction
Hydrogen gas (H₂) doesn't directly react with either water or sodium hydroxide under normal conditions. To understand its indirect role, we need to explore redox reactions. Hydrogen gas can participate in redox reactions, either as a reducing agent (donating electrons) or, less commonly, as an oxidizing agent (accepting electrons). In the context of H₂O and NaOH, H₂'s involvement typically arises from other reactions producing it. For example, the reaction between a strong acid and a strong base will produce salt and water, but if the reaction involves a metal and an acid, H₂ gas might be released.
Indirect Interactions: Electrolysis and Production of Hydrogen
One significant indirect interaction between H₂O, NaOH, and H₂ occurs during the electrolysis of water. Electrolysis is a process that uses an electric current to drive a non-spontaneous chemical reaction. In the presence of an electrolyte like NaOH, the electrolysis of water produces hydrogen gas at the cathode (negative electrode) and oxygen gas at the anode (positive electrode).
The reaction at the cathode is a reduction reaction:
2H₂O(l) + 2e⁻ → H₂(g) + 2OH⁻(aq)
The NaOH acts as an electrolyte, improving the conductivity of water, facilitating the passage of electric current and thus enhancing the efficiency of the electrolysis process. The increased hydroxide ion concentration from the dissolved NaOH doesn't directly participate in the H₂ production but assists in the electrochemical process. The oxygen gas produced at the anode is formed through oxidation:
2H₂O(l) → O₂(g) + 4H⁺(aq) + 4e⁻
The overall reaction for the electrolysis of water using NaOH as an electrolyte can be summarized as:
2H₂O(l) → 2H₂(g) + O₂(g)
This reaction demonstrates how, while NaOH doesn't directly react with H₂, it plays a vital role in its production through electrolysis.
Equilibrium and Le Chatelier's Principle
The concepts of equilibrium and Le Chatelier's principle are important when considering the interplay between H₂O, NaOH, and H₂ in a system. While H₂ doesn't directly react with NaOH or H₂O, its presence might affect the equilibrium of other reactions within the system. For instance, if H₂ is introduced into a system containing water and NaOH, it might influence the equilibrium of reactions involving dissolved gases or if other reactions producing H₂ are present. Le Chatelier's principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. This could involve changes in pressure, temperature, or concentration of reactants or products.
Practical Applications: Industrial and Everyday Relevance
The interactions between H₂O, NaOH, and H₂ have significant practical applications:
-
Electrolysis of Water for Hydrogen Production: The production of hydrogen gas through electrolysis is a crucial method for obtaining clean and sustainable energy. The use of NaOH as an electrolyte is important for efficiency in this process.
-
Chlor-Alkali Process: This industrial process uses an electrolytic cell to produce chlorine gas (Cl₂) and sodium hydroxide (NaOH) from brine (aqueous solution of sodium chloride, NaCl). Hydrogen gas is a byproduct of this process.
-
Soap Making: Sodium hydroxide is a key component in the saponification process, which involves reacting fats and oils with NaOH to produce soap. The reaction generates glycerol as a byproduct.
Frequently Asked Questions (FAQ)
Q1: Can hydrogen gas react directly with sodium hydroxide?
A1: No, under normal conditions, hydrogen gas does not directly react with sodium hydroxide. It requires specific conditions and catalysts for any reaction to occur.
Q2: Is the reaction between sodium hydroxide and water reversible?
A2: The dissociation of sodium hydroxide in water is essentially irreversible under normal conditions. The strong interaction between water molecules and the Na⁺ and OH⁻ ions prevents the reformation of solid NaOH.
Q3: How does the concentration of NaOH affect the electrolysis of water?
A3: A higher concentration of NaOH increases the conductivity of the water, allowing for a more efficient passage of electric current and thus a faster production of hydrogen and oxygen gases.
Q4: What are the safety precautions when working with sodium hydroxide and hydrogen gas?
A4: Sodium hydroxide is a corrosive substance, and it’s crucial to handle it with appropriate safety equipment, such as gloves and eye protection. Hydrogen gas is flammable and can form explosive mixtures with air; proper ventilation is crucial when handling it.
Q5: Are there any other methods of producing hydrogen gas besides electrolysis?
A5: Yes, several other methods exist for hydrogen gas production, including steam methane reforming (SMR), coal gasification, and biomass gasification.
Conclusion: A Complex Interplay of Chemical Principles
The relationship between water, sodium hydroxide, and hydrogen gas reveals a fascinating interplay of fundamental chemical principles. While hydrogen gas doesn't directly interact with sodium hydroxide or water under normal circumstances, its production is often intertwined with these substances, particularly through electrochemical processes like the electrolysis of water. Understanding the dissociation of NaOH in water, the role of electrolysis, and the principles of equilibrium are key to grasping the complex interactions within this chemical trio. The practical applications of these interactions range from industrial-scale production of hydrogen and chlorine to everyday processes like soap making, highlighting the importance of understanding these fundamental chemical relationships. Further research into catalytic reactions and the exploration of new methods for hydrogen production will continue to expand our knowledge of this important chemical interplay.
Latest Posts
Latest Posts
-
Lewis Dot Structure For No
Sep 12, 2025
-
Type Of Bonding In Hcl
Sep 12, 2025
-
X 3 3x 1 0
Sep 12, 2025
-
Fe2o3 Co Fe Co2 Balanced
Sep 12, 2025
-
What Is 1 20 Slope
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Balance Na H2o Naoh H2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.