Fe2o3 Co Fe Co2 Balanced
thesills
Sep 12, 2025 · 6 min read
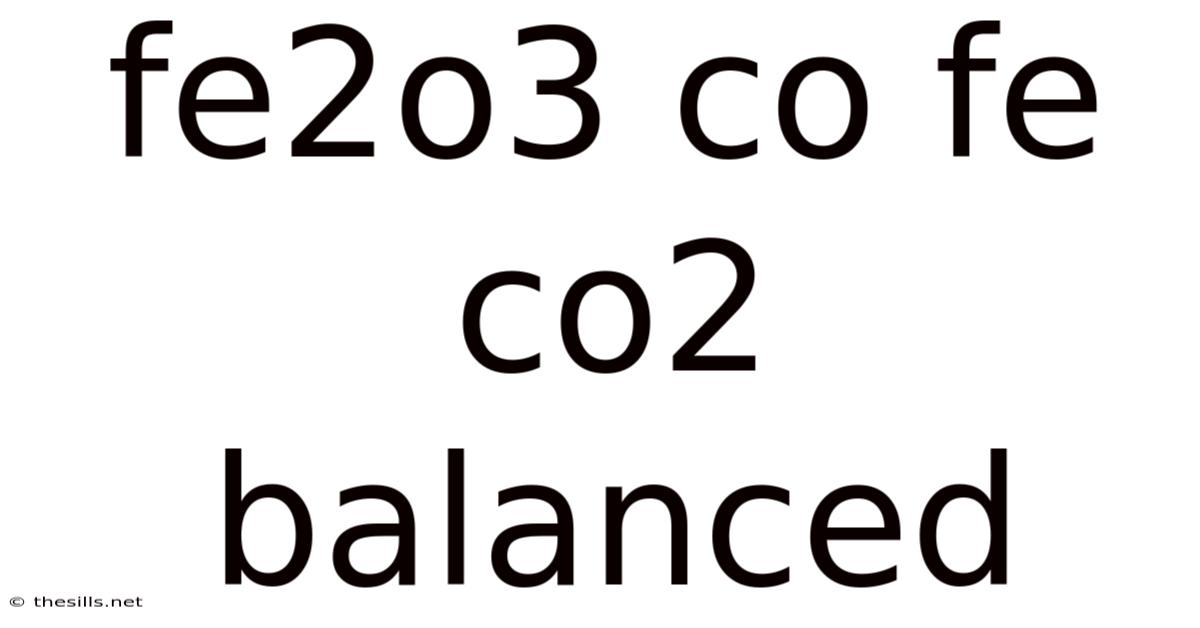
Table of Contents
Understanding the Balanced Equation: Fe₂O₃ + CO → Fe + CO₂
The reaction between iron(III) oxide (Fe₂O₃) and carbon monoxide (CO) to produce iron (Fe) and carbon dioxide (CO₂) is a fundamental chemical process with significant industrial applications, particularly in iron smelting. Understanding this reaction, including its balanced equation and the underlying chemistry, is crucial for various fields, from materials science to environmental engineering. This article delves deep into the Fe₂O₃ + CO → Fe + CO₂ reaction, exploring its balanced equation, the step-by-step process, the scientific principles involved, and frequently asked questions.
Introduction: The Heart of Iron Production
The reaction between iron(III) oxide and carbon monoxide forms the basis of the blast furnace process, a cornerstone of iron and steel production. In this high-temperature process, iron ore (which contains Fe₂O₃) is reduced to metallic iron using CO as a reducing agent. The reaction is exothermic, meaning it releases heat, contributing to the overall energy efficiency of the process. This reaction isn't simply a classroom exercise; it’s a vital industrial process impacting global manufacturing and infrastructure. Understanding its intricacies provides a window into the chemistry behind one of humanity's most important materials.
Balancing the Equation: A Step-by-Step Guide
Before diving into the details, let's establish the balanced chemical equation. An unbalanced equation simply shows the reactants and products:
Fe₂O₃ + CO → Fe + CO₂
This equation is unbalanced because the number of atoms of each element is not equal on both sides of the arrow. To balance it, we need to adjust the coefficients (the numbers in front of each chemical formula) to ensure that the number of atoms of each element is the same on both the reactant and product sides.
Here's the step-by-step process to balance the equation:
- Iron (Fe): We start with iron. There are two iron atoms on the reactant side (in Fe₂O₃) and only one on the product side. To balance this, we place a coefficient of 2 in front of Fe:
Fe₂O₃ + CO → 2Fe + CO₂
- Oxygen (O): Next, let's look at oxygen. There are three oxygen atoms on the reactant side (one in CO and two in Fe₂O₃) and two on the product side. We need to find coefficients that will balance the oxygen atoms. Let's try placing a coefficient of 3 in front of CO₂:
Fe₂O₃ + CO → 2Fe + 3CO₂
Now, we have three oxygen atoms on the product side. However, this changes the number of carbon atoms.
- Carbon (C) and Oxygen (O): We now have three carbon atoms on the product side. To balance this, we must place a coefficient of 3 in front of CO on the reactant side:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
Now let's check the oxygen atoms again. We have three oxygen atoms from Fe₂O₃ + (3 x 1) oxygen atoms from 3CO = 6 oxygen atoms on the reactant side. And we have (3 x 2) = 6 oxygen atoms on the product side. The oxygen atoms are balanced.
The balanced chemical equation is:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
This equation now shows that one molecule of iron(III) oxide reacts with three molecules of carbon monoxide to produce two atoms of iron and three molecules of carbon dioxide. The number of atoms of each element is equal on both sides of the equation.
The Scientific Explanation: Reduction and Oxidation
This reaction is a classic example of a redox reaction, short for reduction-oxidation reaction. Redox reactions involve the transfer of electrons between atoms.
-
Reduction: In this reaction, iron(III) ions (Fe³⁺) in Fe₂O₃ gain electrons and are reduced to neutral iron atoms (Fe). The oxidation state of iron changes from +3 to 0. Carbon monoxide acts as the reducing agent, donating electrons to the iron.
-
Oxidation: Simultaneously, carbon atoms in CO lose electrons and are oxidized to form carbon dioxide (CO₂). The oxidation state of carbon changes from +2 to +4. Iron(III) oxide acts as the oxidizing agent, accepting electrons from the carbon.
The overall reaction can be viewed as a competition for electrons between iron and carbon. Carbon monoxide, having a slightly lower electronegativity than iron, is more willing to donate its electrons, thus reducing the iron.
The process is highly temperature-dependent. The high temperatures in a blast furnace provide the necessary activation energy for the reaction to proceed at a commercially viable rate.
Step-by-Step Process in a Blast Furnace
The industrial application of this reaction within a blast furnace is a complex, multi-step process:
-
Charging: Iron ore (containing Fe₂O₃), coke (a form of carbon), and limestone (CaCO₃) are charged into the top of the blast furnace.
-
Preheating: Hot air is blown into the bottom of the furnace, preheating the materials.
-
Combustion: The coke reacts with the hot air, producing carbon monoxide (CO) and releasing significant heat: 2C + O₂ → 2CO
-
Reduction: The hot carbon monoxide then rises through the furnace, reacting with the iron ore (Fe₂O₃) according to the balanced equation we discussed: Fe₂O₃ + 3CO → 2Fe + 3CO₂
-
Slag Formation: Limestone reacts with impurities in the iron ore, forming slag (a molten mixture of calcium silicates), which is then removed from the furnace.
-
Iron Collection: Molten iron collects at the bottom of the furnace and is tapped off periodically.
Frequently Asked Questions (FAQs)
Q1: What are the conditions required for this reaction to occur efficiently?
A1: High temperatures (around 1500-2000°C) and the presence of sufficient carbon monoxide are crucial for efficient reaction. The reaction rate increases dramatically with temperature.
Q2: Is this reaction reversible?
A2: While theoretically reversible, in practice, the reaction proceeds almost entirely to completion under blast furnace conditions due to the high temperatures and removal of products.
Q3: What are the industrial applications of this reaction?
A3: The primary application is in the production of iron and steel. It's the cornerstone of the entire iron and steel industry.
Q4: Are there any environmental concerns associated with this reaction?
A4: The process produces significant amounts of carbon dioxide, a greenhouse gas contributing to climate change. Research is ongoing to develop more sustainable iron production methods.
Q5: Can other reducing agents be used instead of carbon monoxide?
A5: Yes, other reducing agents, such as hydrogen (H₂), can theoretically reduce iron(III) oxide. However, carbon monoxide is currently the most cost-effective and widely used reducing agent in industrial iron production.
Conclusion: A Reaction with Global Impact
The seemingly simple reaction between iron(III) oxide and carbon monoxide, represented by the balanced equation Fe₂O₃ + 3CO → 2Fe + 3CO₂, is a cornerstone of modern industrial processes. Understanding this reaction – its balanced equation, the underlying redox chemistry, and its industrial application within the blast furnace – provides valuable insight into the production of one of the world's most vital materials. While environmental considerations related to CO₂ emissions are important, ongoing research and technological advancements continuously strive to improve the sustainability of this vital chemical process. This reaction isn't just a chemical equation; it's a story of human ingenuity and the continuous drive to improve upon fundamental chemical processes.
Latest Posts
Latest Posts
-
Difference Between Microscope And Telescope
Sep 12, 2025
-
Copper Wiring Vs Silver Wiring
Sep 12, 2025
-
Sentence Using The Word Constitution
Sep 12, 2025
-
Indefinite Shape And Indefinite Volume
Sep 12, 2025
-
What Is 2x Times 3x
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Fe2o3 Co Fe Co2 Balanced . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.