Magnesium Iodine Gives Magnesium Iodide
thesills
Sep 15, 2025 · 6 min read
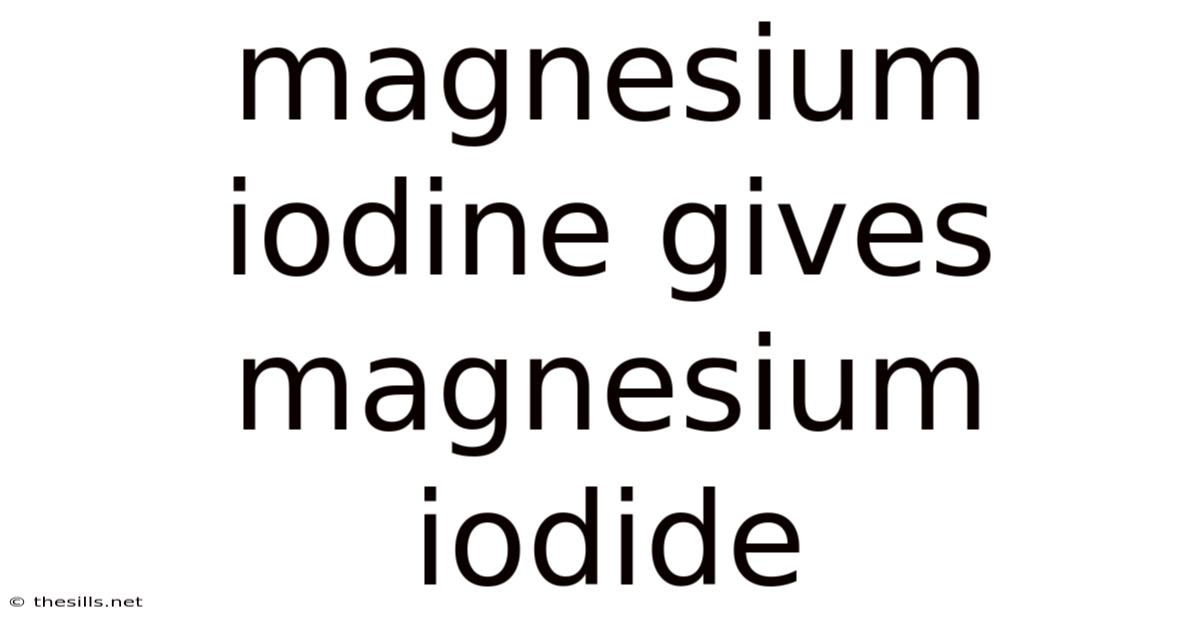
Table of Contents
Magnesium and Iodine: A Deep Dive into the Formation of Magnesium Iodide
Magnesium iodide, a seemingly simple chemical compound, holds a fascinating place in the world of chemistry. Understanding its formation from magnesium and iodine provides a window into fundamental chemical principles, including redox reactions, ionic bonding, and the reactivity of alkali earth metals and halogens. This article will delve into the intricacies of this reaction, exploring the process, the scientific principles involved, practical applications, safety considerations, and frequently asked questions.
Introduction: The Reaction Between Magnesium and Iodine
The reaction between magnesium (Mg), a silvery-white alkaline earth metal, and iodine (I₂), a dark purplish-black nonmetal, is a classic example of a redox reaction, specifically a single displacement or substitution reaction. It results in the formation of magnesium iodide (MgI₂), a white crystalline solid. This seemingly straightforward reaction provides a rich learning opportunity to understand fundamental concepts in chemistry and the behavior of elements within their respective groups on the periodic table. The reaction is exothermic, meaning it releases heat, which can be observed as an increase in temperature during the reaction. The key to understanding this reaction lies in the difference in electronegativity between magnesium and iodine.
Understanding the Chemical Principles Involved
Redox Reactions (Oxidation-Reduction): At the heart of the magnesium-iodine reaction lies a redox reaction. Magnesium is oxidized, meaning it loses electrons, while iodine is reduced, meaning it gains electrons. This electron transfer is crucial for the formation of the ionic bond between magnesium and iodine.
-
Oxidation: Magnesium, with its two valence electrons, readily loses these electrons to achieve a stable electron configuration resembling that of the noble gas neon. This process is represented by the following half-reaction:
Mg → Mg²⁺ + 2e⁻
-
Reduction: Iodine, a diatomic molecule (I₂), gains one electron per atom to achieve a stable electron configuration resembling that of the noble gas xenon. This process is represented by the following half-reaction:
I₂ + 2e⁻ → 2I⁻
By combining these two half-reactions, we obtain the overall balanced redox reaction:
Mg + I₂ → MgI₂
Ionic Bonding: The resulting magnesium iodide is an ionic compound. This means that the magnesium atoms lose electrons to become positively charged ions (Mg²⁺ cations), and the iodine atoms gain electrons to become negatively charged ions (I⁻ anions). The electrostatic attraction between these oppositely charged ions forms the ionic bond, holding the crystal lattice structure of magnesium iodide together. The strong electrostatic forces contribute to the high melting and boiling points of ionic compounds like magnesium iodide.
Reactivity of Magnesium and Iodine: Magnesium's position in Group 2 of the periodic table indicates its tendency to lose two electrons and form a +2 ion. Iodine, in Group 17 (halogens), readily accepts one electron to form a -1 ion. This difference in reactivity drives the spontaneous reaction between magnesium and iodine. The high electronegativity of iodine further contributes to its ability to attract electrons from magnesium.
Steps to Perform the Reaction (Laboratory Setting)
While this reaction can be demonstrated in a simple laboratory setting, safety precautions must be rigorously followed. Iodine is a corrosive substance, and the reaction can generate heat. Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat. The reaction should be performed under the supervision of a qualified instructor.
-
Preparation: Obtain small pieces of magnesium ribbon (cleaned with sandpaper to remove any oxide layer) and iodine crystals. It's crucial to handle iodine carefully to avoid skin contact or inhalation of its vapor. Use a clean, dry watch glass or evaporating dish.
-
Mixing: Gently place a small amount of iodine crystals on the watch glass. Add a small piece of magnesium ribbon to the iodine. You may need to lightly crush the magnesium ribbon to increase the surface area.
-
Reaction Initiation: The reaction may require some initiation, typically a gentle warming or the addition of a small amount of water (with caution). The reaction is relatively slow at room temperature but will accelerate with slight warming. You'll observe the magnesium ribbon slowly reacting with the iodine, producing a white magnesium iodide solid. The reaction is accompanied by the release of heat.
-
Observation: Observe the color change from the dark purple iodine to the white magnesium iodide. The reaction might proceed with a slight glow as heat is released. The reaction is typically slower than other similar reactions with more reactive halogens such as chlorine or bromine.
-
Cleanup: After the reaction has completed, carefully dispose of the magnesium iodide waste according to the appropriate safety protocols of your institution.
Practical Applications of Magnesium Iodide
Magnesium iodide finds applications in various fields, although it is not as widely used as some other magnesium salts:
-
Synthesis of other compounds: It serves as a precursor in the synthesis of various organomagnesium compounds, which are useful in organic chemistry.
-
Grignard Reagents: While not directly used, the principle behind the formation of magnesium iodide is essential in the formation of Grignard reagents (organomagnesium halides), powerful tools for carbon-carbon bond formation in organic synthesis. The reaction of magnesium with alkyl halides in an ether solvent forms Grignard reagents.
-
Medicine (Potential Application): Research explores its potential use in medicine, particularly in areas related to its iodide ion content. Iodine is an essential element for thyroid hormone production, but further research is needed to determine its precise medicinal applications.
Safety Precautions
- Iodine is corrosive and can stain skin and clothing. Wear appropriate PPE at all times.
- The reaction generates heat. Avoid direct contact and perform the reaction in a well-ventilated area.
- Iodine vapor is irritating to the respiratory system. Work in a fume hood if available or in a well-ventilated area.
- Dispose of waste properly. Follow institutional guidelines for chemical waste disposal.
Frequently Asked Questions (FAQs)
-
Q: Is the reaction between magnesium and iodine explosive?
- A: No, the reaction is not explosive under normal laboratory conditions. However, it is exothermic and releases heat.
-
Q: Why is the reaction slower than that of magnesium with chlorine or bromine?
- A: Iodine is less reactive than chlorine or bromine. This is due to its larger atomic size and lower electronegativity, making it less effective in attracting and accepting electrons from magnesium.
-
Q: Can the reaction be accelerated?
- A: Yes, the reaction can be accelerated by increasing the surface area of the magnesium (using magnesium powder instead of ribbon) or slightly warming the reactants. The addition of a small amount of water can also initiate the reaction, however, this should be approached with caution and only under appropriate supervision.
-
Q: What are the physical properties of magnesium iodide?
- A: Magnesium iodide is a white crystalline solid. It is hygroscopic, meaning it readily absorbs moisture from the air. It is soluble in water and other polar solvents.
Conclusion: A Fundamental Reaction with Broad Implications
The reaction between magnesium and iodine to form magnesium iodide is a deceptively simple yet profoundly instructive chemical reaction. It serves as a cornerstone for understanding fundamental concepts in chemistry, including redox reactions, ionic bonding, and the periodic trends in reactivity. While magnesium iodide itself might not have widespread direct applications compared to other magnesium compounds, its formation is critical to understanding the principles behind many crucial chemical processes, particularly in organic synthesis. The careful study of this reaction provides a solid foundation for further exploration of the fascinating world of chemical reactions and their applications. Through understanding the principles of this reaction, we gain a deeper appreciation for the elegance and power of chemical transformations. Remember to always prioritize safety when conducting any chemical experiments.
Latest Posts
Latest Posts
-
Cast Iron Modulus Of Elasticity
Sep 15, 2025
-
6 4 As A Mixed Number
Sep 15, 2025
-
Dynamometer Is Used To Measure
Sep 15, 2025
-
Can A Solid Be Compressed
Sep 15, 2025
-
Grams To Atoms Conversion Calculator
Sep 15, 2025
Related Post
Thank you for visiting our website which covers about Magnesium Iodine Gives Magnesium Iodide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.