Kno3 H2co3 K2co3 Hno3 Balanced
thesills
Sep 16, 2025 · 6 min read
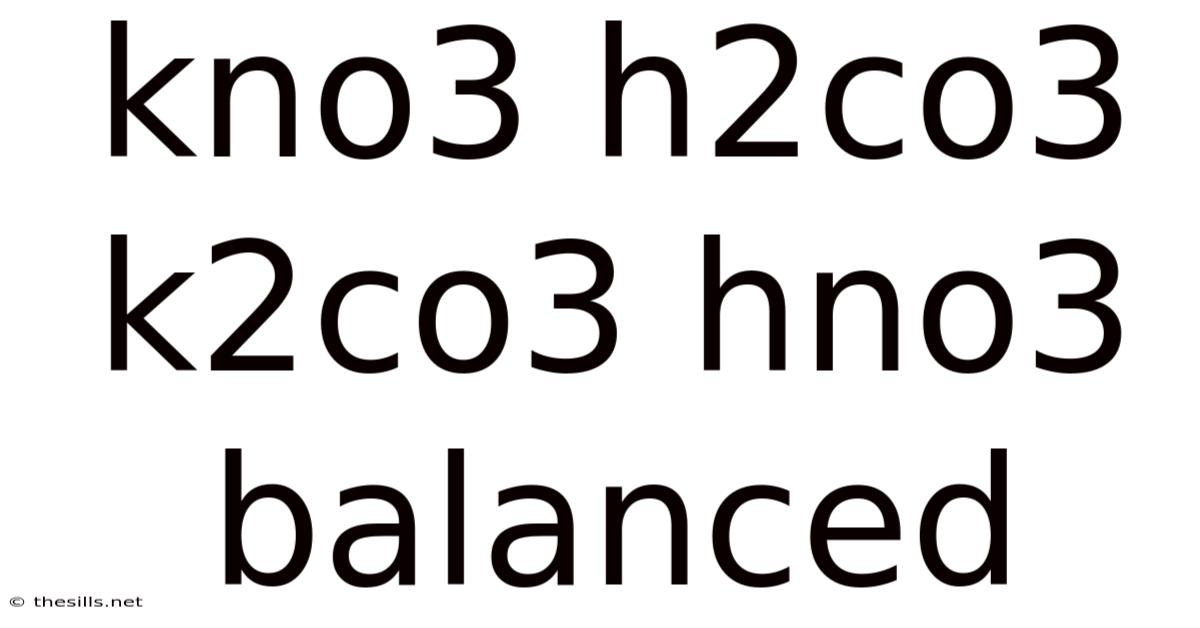
Table of Contents
Balancing Chemical Equations: A Deep Dive into KNO3, H2CO3, K2CO3, and HNO3
Understanding how to balance chemical equations is fundamental to comprehending chemical reactions. This article will provide a comprehensive guide on balancing chemical equations, focusing specifically on the compounds potassium nitrate (KNO₃), carbonic acid (H₂CO₃), potassium carbonate (K₂CO₃), and nitric acid (HNO₃). We'll explore their individual properties, common reactions, and the step-by-step process of balancing equations involving these compounds. This will equip you with a solid foundation in stoichiometry and chemical calculations.
Introduction: The Importance of Balanced Equations
Chemical equations represent the symbolic representation of chemical reactions. A balanced chemical equation ensures the law of conservation of mass is obeyed; meaning the number of atoms of each element remains the same on both the reactant and product sides of the equation. Unbalanced equations are incomplete and don't accurately reflect the quantities of substances involved in a reaction. Mastering the art of balancing equations is crucial for performing accurate stoichiometric calculations, predicting reaction yields, and understanding the quantitative aspects of chemistry. This is especially important when dealing with compounds like KNO₃, H₂CO₃, K₂CO₃, and HNO₃ which are involved in a variety of important chemical processes.
Understanding the Compounds Involved
Before diving into balancing equations, let's briefly review the properties of each compound:
-
Potassium Nitrate (KNO₃): A crystalline salt commonly used in fertilizers, gunpowder, and food preservation (as a preservative, E number E252). It's a strong oxidizing agent.
-
Carbonic Acid (H₂CO₃): A weak diprotic acid formed when carbon dioxide dissolves in water. It's unstable and readily decomposes into water and carbon dioxide.
-
Potassium Carbonate (K₂CO₃): A white crystalline salt, also known as potash. It's used in the production of glass, detergents, and other chemicals. It's a base.
-
Nitric Acid (HNO₃): A strong, highly corrosive acid. It's used extensively in the production of fertilizers, explosives, and other chemicals.
Balancing Equations: A Step-by-Step Approach
Balancing chemical equations involves adjusting the coefficients (numbers placed before the chemical formulas) to ensure that the number of atoms of each element is equal on both sides of the equation. There isn't a single, universal method, but a systematic approach is key. Here's a breakdown of a common approach:
-
Write the Unbalanced Equation: Begin by writing the chemical equation with the reactants on the left side and the products on the right side, separated by an arrow. For example, let's consider a reaction involving potassium nitrate and carbonic acid:
KNO₃ + H₂CO₃ → K₂CO₃ + HNO₃
-
Identify the Elements: List all the elements present in the equation: Potassium (K), Nitrogen (N), Oxygen (O), Hydrogen (H), and Carbon (C).
-
Count the Atoms: Count the number of atoms of each element on both the reactant and product sides.
- Reactants: K: 1, N: 1, O: 6, H: 2, C: 1
- Products: K: 2, N: 1, O: 6, H: 1, C: 1
-
Balance the Equation: Start by balancing the element that appears in the most complex molecule. We can start with Potassium (K). To balance the potassium atoms, we place a coefficient of 2 in front of KNO₃ on the reactant side:
2KNO₃ + H₂CO₃ → K₂CO₃ + HNO₃
Now let's recount the atoms:
- Reactants: K: 2, N: 2, O: 8, H: 2, C: 1
- Products: K: 2, N: 1, O: 6, H: 1, C: 1
Notice that the nitrogen (N) is now unbalanced. To balance the nitrogen atoms, add a coefficient of 2 in front of HNO₃ on the product side:
2KNO₃ + H₂CO₃ → K₂CO₃ + 2HNO₃
Recounting the atoms:
- Reactants: K: 2, N: 2, O: 8, H: 2, C: 1
- Products: K: 2, N: 2, O: 8, H: 2, C: 1
The equation is now balanced! All elements have the same number of atoms on both sides.
More Complex Examples and Strategies
Let's consider another example involving a reaction between potassium carbonate and nitric acid:
K₂CO₃ + HNO₃ → KNO₃ + H₂O + CO₂
-
Unbalanced Equation: K₂CO₃ + HNO₃ → KNO₃ + H₂O + CO₂
-
Count Atoms: Start by counting the atoms of each element on both sides.
-
Balance: This equation requires a more strategic approach. Let's start by balancing the potassium (K) atoms. We need two potassium atoms on the product side, so we place a coefficient of 2 in front of KNO₃:
K₂CO₃ + HNO₃ → 2KNO₃ + H₂O + CO₂
Now, balance the nitrogen (N) atoms. There are two nitrogen atoms on the product side, so we need to add a coefficient of 2 in front of HNO₃ on the reactant side:
K₂CO₃ + 2HNO₃ → 2KNO₃ + H₂O + CO₂
Finally, check the hydrogen (H) and oxygen (O) atoms. They are already balanced.
Therefore, the balanced equation is: K₂CO₃ + 2HNO₃ → 2KNO₃ + H₂O + CO₂
Strategies for Balancing Difficult Equations:
- Start with the most complex molecule: Begin balancing the element present in the most complex molecule to simplify the process.
- Balance polyatomic ions as a unit: If polyatomic ions (like NO₃⁻ or CO₃²⁻) remain unchanged throughout the reaction, treat them as a single unit during balancing.
- Balance elements that appear only once on each side first: This often simplifies the process.
- Use fractional coefficients if necessary: You can use fractional coefficients as intermediate steps, but ultimately convert them to whole numbers by multiplying the entire equation by the appropriate factor.
- Check your work: Always recount the atoms on both sides after each step to ensure accuracy.
Further Applications and Considerations
Balancing chemical equations is not just an academic exercise. It has crucial applications in various fields, including:
- Stoichiometry: Predicting the amounts of reactants and products in a chemical reaction.
- Industrial Chemistry: Optimizing chemical processes and determining reaction yields in industrial settings.
- Environmental Chemistry: Analyzing and modeling chemical reactions in the environment.
- Analytical Chemistry: Performing quantitative analysis of chemical samples.
Frequently Asked Questions (FAQ)
-
Q: What happens if I get stuck balancing an equation?
- A: Try a different element to start with, or use fractional coefficients as an intermediate step. If still stuck, double-check your initial equation and atom count.
-
Q: Are there any online tools or software to help balance equations?
- A: Yes, many online tools and software programs are available to help balance chemical equations. However, understanding the underlying principles is essential.
-
Q: Why is it important to balance equations accurately?
- A: An unbalanced equation doesn't represent the actual reaction accurately and leads to incorrect stoichiometric calculations and predictions.
Conclusion:
Balancing chemical equations is a fundamental skill in chemistry. By following a systematic approach and utilizing the strategies discussed above, you can successfully balance even complex chemical equations. This skill is vital for understanding chemical reactions, performing stoichiometric calculations, and solving problems in various scientific and industrial applications involving compounds like KNO₃, H₂CO₃, K₂CO₃, and HNO₃. Remember practice makes perfect. The more equations you balance, the more proficient and confident you'll become.
Latest Posts
Latest Posts
-
Where Are Glycosidic Bonds Found
Sep 16, 2025
-
When Was Sulfuric Acid Discovered
Sep 16, 2025
-
Plot Ln K Vs 1 T
Sep 16, 2025
-
Is Lioh A Strong Acid
Sep 16, 2025
-
Find The Volume Of Parallelepiped
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about Kno3 H2co3 K2co3 Hno3 Balanced . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.