Plot Ln K Vs 1/t
thesills
Sep 16, 2025 · 7 min read
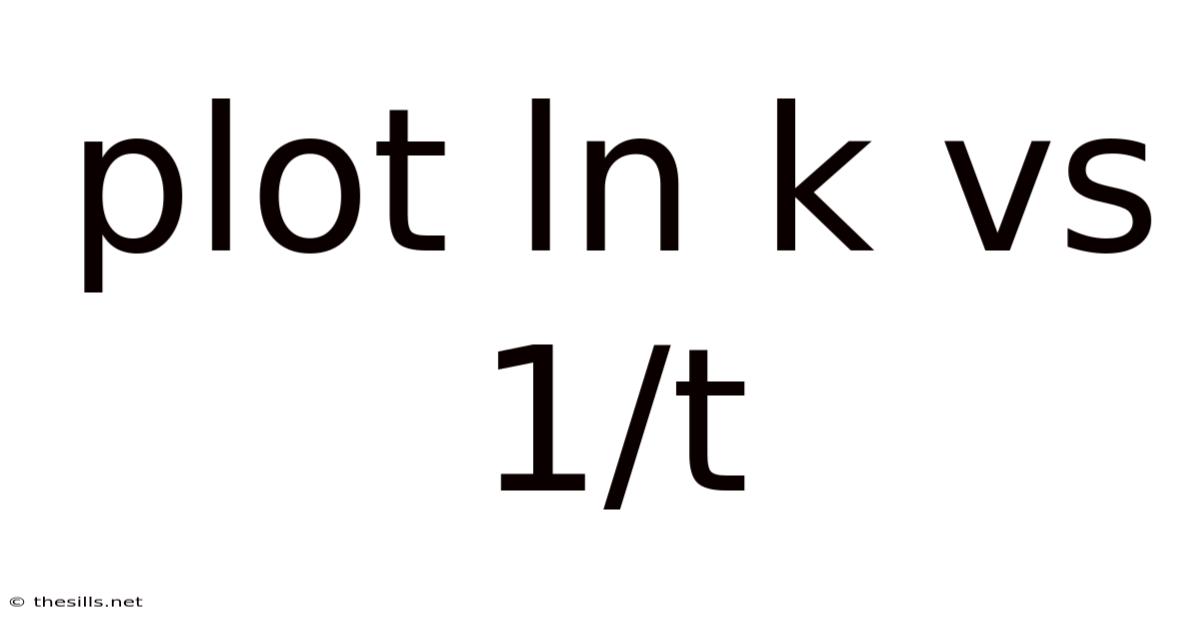
Table of Contents
Unveiling Reaction Mechanisms: A Deep Dive into Plotting ln k vs 1/T
Understanding the kinetics of chemical reactions is fundamental to numerous fields, from industrial chemical engineering to biological processes. One powerful tool for analyzing reaction rates and determining activation energies is the Arrhenius equation, and its graphical representation through a plot of ln k versus 1/T. This article provides a comprehensive exploration of this technique, explaining its theoretical underpinnings, practical applications, and potential pitfalls. We will delve into the significance of the slope and intercept, discuss how to perform the plot, and address frequently asked questions. By the end, you'll have a solid understanding of how to use this powerful tool to gain insights into reaction mechanisms.
Introduction: The Arrhenius Equation and its Significance
The rate of a chemical reaction, denoted by k, is rarely constant; it's heavily influenced by temperature. The Arrhenius equation elegantly quantifies this relationship:
k = A * exp(-Ea/RT)
Where:
- k is the rate constant of the reaction
- A is the pre-exponential factor (frequency factor), representing the frequency of collisions with the correct orientation.
- Ea is the activation energy, the minimum energy required for the reaction to occur.
- R is the ideal gas constant (8.314 J/mol·K)
- T is the absolute temperature in Kelvin.
This equation reveals that the rate constant k increases exponentially with temperature. However, its non-linear nature makes it challenging to directly interpret experimental data. To linearize the equation and facilitate data analysis, we take the natural logarithm of both sides:
ln k = ln A - Ea/RT
This transformed equation now takes the form of a linear equation, y = mx + c, where:
- y = ln k
- x = 1/T
- m = -Ea/R (slope)
- c = ln A (y-intercept)
This linear relationship is the key to plotting ln k vs 1/T. By plotting the natural logarithm of the rate constant against the reciprocal of the absolute temperature, we obtain a straight line, allowing for a straightforward determination of the activation energy and pre-exponential factor.
Plotting ln k vs 1/T: A Step-by-Step Guide
Creating an accurate and informative ln k vs 1/T plot requires careful attention to detail. Here's a step-by-step guide:
-
Gather Experimental Data: You'll need a series of rate constants (k) measured at different temperatures (T). Ensure your temperature readings are precise and accurate, and that your rate constants are determined using a reliable experimental method. The more data points you have, the more reliable your plot will be.
-
Convert Temperature to Kelvin: Remember, the Arrhenius equation requires absolute temperature. Convert all your Celsius or Fahrenheit readings to Kelvin using the appropriate formula (K = °C + 273.15).
-
Calculate the Reciprocal of Temperature (1/T): For each temperature value in Kelvin, calculate its reciprocal (1/T). This will be the x-axis value for your plot.
-
Calculate the Natural Logarithm of the Rate Constant (ln k): For each rate constant (k) you've measured, calculate its natural logarithm (ln k). This will be the y-axis value for your plot.
-
Create the Plot: Using graphing software or even graph paper, plot ln k (y-axis) against 1/T (x-axis). Each data point represents a single experimental measurement.
-
Draw the Best-Fit Line: The data points should ideally fall along a straight line. Use linear regression (least-squares method) to determine the best-fit line that minimizes the sum of the squared deviations between the data points and the line. Many graphing programs will do this automatically.
-
Determine the Slope and Intercept: The slope of the best-fit line is equal to -Ea/R, and the y-intercept is equal to ln A. Use these values to calculate the activation energy (Ea) and pre-exponential factor (A).
Determining Activation Energy (Ea) and Pre-exponential Factor (A)
Once you have the slope and intercept from your ln k vs 1/T plot, calculating the activation energy and pre-exponential factor is straightforward:
-
Activation Energy (Ea): The slope of the line (m) is equal to -Ea/R. Therefore, Ea = -m * R. Remember to use the correct units for R (8.314 J/mol·K) to obtain Ea in Joules per mole (J/mol). It's often more convenient to express Ea in kilojoules per mole (kJ/mol) by dividing by 1000.
-
Pre-exponential Factor (A): The y-intercept (c) is equal to ln A. Therefore, A = exp(c). The pre-exponential factor represents the frequency of successful collisions with the correct orientation.
Interpreting the Plot and its Implications
The ln k vs 1/T plot provides valuable insights into the reaction kinetics:
-
Activation Energy (Ea): A high activation energy indicates that a significant amount of energy is required for the reaction to proceed, meaning the reaction will be slower at lower temperatures. A lower activation energy suggests a faster reaction at lower temperatures. The magnitude of Ea can offer clues about the reaction mechanism, with higher values often suggesting more complex mechanisms.
-
Pre-exponential Factor (A): The pre-exponential factor reflects the frequency of collisions and the probability of those collisions leading to a successful reaction. A higher A value indicates a higher probability of a successful reaction.
-
Linearity: A perfectly linear plot indicates that the Arrhenius equation accurately describes the reaction kinetics over the temperature range studied. Deviations from linearity may suggest a change in the reaction mechanism or other complicating factors.
-
Comparing Reactions: By comparing the ln k vs 1/T plots for different reactions, you can compare their activation energies and pre-exponential factors, offering insights into their relative rates and mechanisms.
Potential Pitfalls and Considerations
While plotting ln k vs 1/T is a powerful technique, several potential pitfalls must be addressed:
-
Temperature Range: The Arrhenius equation assumes a constant activation energy over the temperature range studied. Significant deviations from linearity might suggest a change in the mechanism at higher or lower temperatures. Restricting your analysis to a narrower, more appropriate range might be necessary.
-
Experimental Error: Experimental errors in measuring both the rate constants and temperatures can significantly affect the accuracy of the slope and intercept. Proper experimental design and error analysis are crucial. Consider repeating experiments at each temperature to improve the reliability of your data.
-
Complex Reactions: The Arrhenius equation is best suited for simple, elementary reactions. For complex reactions involving multiple steps, the interpretation of the activation energy and pre-exponential factor can be more challenging.
-
Non-Arrhenius Behavior: Some reactions exhibit non-Arrhenius behavior, meaning their rate constants do not follow the simple exponential relationship described by the Arrhenius equation. This often suggests a more complex reaction mechanism or other factors influencing the reaction rate.
Frequently Asked Questions (FAQ)
-
Q: What if my plot isn't perfectly linear?
- A: Non-linearity can indicate a change in the reaction mechanism over the temperature range studied, experimental error, or non-Arrhenius behavior. Carefully examine your data and experimental procedures.
-
Q: What units should I use for activation energy?
- A: Activation energy (Ea) is typically expressed in Joules per mole (J/mol) or kilojoules per mole (kJ/mol).
-
Q: How does the pre-exponential factor (A) relate to the reaction mechanism?
- A: A higher A value suggests a greater frequency of successful collisions and a higher probability of reaction. The value of A can provide insights into the steric factors affecting the reaction.
-
Q: Can I use this method for catalyzed reactions?
- A: Yes, you can use this method for catalyzed reactions, but the activation energy determined will reflect the catalyzed reaction pathway, not the uncatalyzed one.
-
Q: What are the limitations of the Arrhenius equation?
- A: The Arrhenius equation is an empirical equation, meaning it's based on experimental observations rather than a rigorous theoretical derivation. It works well for many reactions but might not accurately describe reactions with complex mechanisms or those exhibiting non-Arrhenius behavior.
Conclusion: A Powerful Tool for Kinetic Analysis
Plotting ln k versus 1/T is a powerful and widely used technique for determining activation energies and pre-exponential factors from experimental kinetic data. By understanding the underlying principles of the Arrhenius equation and carefully following the steps outlined above, you can gain valuable insights into the reaction mechanisms and rate-limiting steps of chemical reactions. Remember to critically evaluate your data and consider potential sources of error. While the method has limitations, it remains a fundamental tool in the study of chemical kinetics and reaction mechanisms across various scientific disciplines. Mastering this technique will greatly enhance your ability to analyze and interpret reaction rate data, enabling a deeper understanding of chemical processes.
Latest Posts
Latest Posts
-
Convert 0 3 Into A Fraction
Sep 16, 2025
-
What Is 12 Of 5000
Sep 16, 2025
-
Impedance Formula For Rlc Circuit
Sep 16, 2025
-
Why Are Plants Considered Producers
Sep 16, 2025
-
Formation Of Urine Flow Chart
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about Plot Ln K Vs 1/t . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.