2 2 4 4 Tetramethylpentane
thesills
Sep 16, 2025 · 6 min read
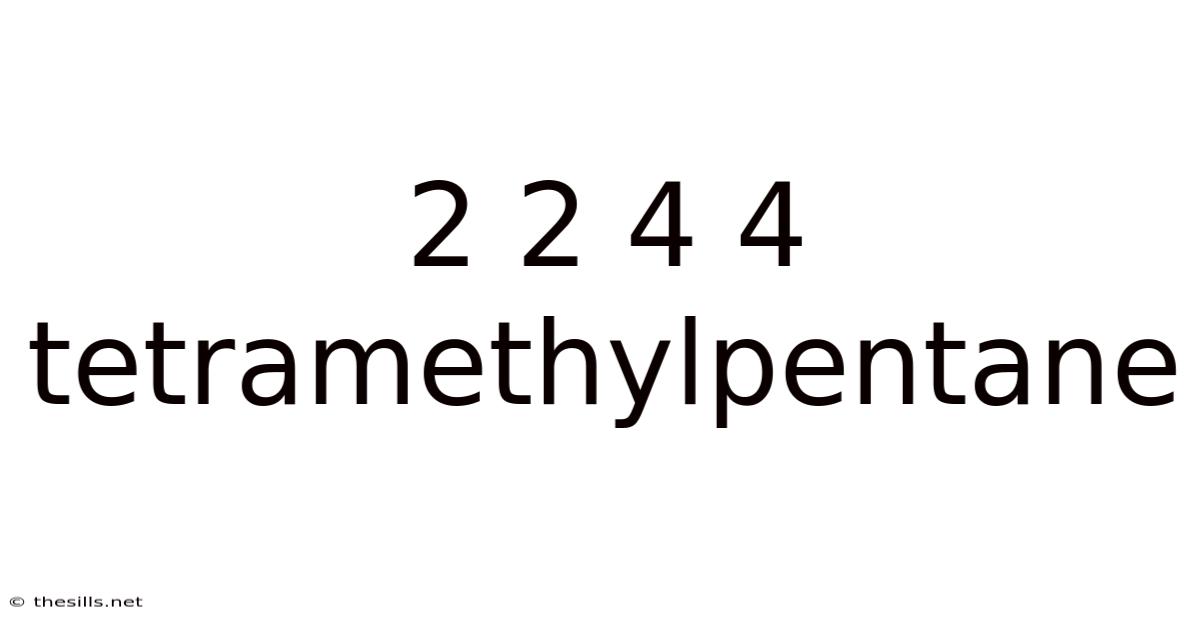
Table of Contents
Decoding 2,2,4,4-Tetramethylpentane: A Deep Dive into Structure, Properties, and Applications
2,2,4,4-Tetramethylpentane, often abbreviated as TMP, is a fascinating organic compound with a deceptively simple chemical formula: C₉H₂₀. Its unique structure and properties have garnered interest in various fields, from fuel chemistry to organic synthesis. This article will delve deep into the world of 2,2,4,4-Tetramethylpentane, exploring its structure, physical and chemical properties, synthesis methods, and applications. We'll also address some frequently asked questions about this intriguing molecule.
Understanding the Structure of 2,2,4,4-Tetramethylpentane
The name itself provides clues to the molecule's structure. "Pentane" signifies a five-carbon chain as the backbone. The prefixes "tetramethyl" indicate the presence of four methyl groups (-CH₃). The numbers 2, 2, 4, and 4 specify the location of these methyl groups on the pentane chain. Therefore, we have two methyl groups attached to the second carbon atom, and two more attached to the fourth carbon atom.
This arrangement creates a highly branched, compact structure. Unlike its linear isomer, n-nonane, TMP's structure significantly impacts its physical and chemical properties. Imagine trying to pack spheres (representing the carbon atoms) together: the branched structure of TMP is far more efficient, leading to a higher density and different reactivity compared to its linear counterparts. This compact structure is crucial in understanding its behaviour as a fuel and its interaction with other molecules.
Physical and Chemical Properties: A Detailed Look
The structural compactness of 2,2,4,4-Tetramethylpentane manifests in several key physical properties:
-
High Octane Rating: This is perhaps the most significant property of TMP. Its branched structure prevents premature ignition in internal combustion engines, leading to a very high octane rating. This makes it a valuable component in high-performance fuels, contributing to smoother engine operation and improved efficiency. The high octane number is directly correlated to the molecule's resistance to knocking, a phenomenon that causes engine damage.
-
Low Boiling Point: While it's a relatively large molecule, TMP's branching leads to a lower boiling point compared to linear alkanes with the same number of carbon atoms. The reduced surface area for intermolecular interactions translates to weaker van der Waals forces, allowing the molecules to escape the liquid phase more readily.
-
Density: TMP displays a relatively high density compared to other nonanes. This again stems from its compact structure, allowing more molecules to be packed into a given volume.
-
Solubility: Like most hydrocarbons, TMP is practically insoluble in water but readily soluble in many organic solvents. This is due to its nonpolar nature and the predominance of nonpolar interactions with other organic molecules.
Chemically, TMP exhibits typical alkane reactivity. While relatively unreactive compared to molecules containing functional groups like alcohols or ketones, it can undergo reactions such as:
-
Combustion: TMP readily burns in the presence of oxygen, producing carbon dioxide and water. This combustion reaction is the basis for its use as a fuel.
-
Halogenation: Under appropriate conditions, TMP can react with halogens (chlorine, bromine) through free radical substitution reactions. This involves replacing one or more hydrogen atoms with halogen atoms.
-
Isomerization: Although not easily achieved under normal conditions, TMP can be isomerized to other nonane isomers under specific catalytic conditions and high temperatures. This is a crucial process in refining petroleum to tailor fuel properties.
Synthesis and Production Methods: From Crude Oil to Pure Compound
The primary source of 2,2,4,4-Tetramethylpentane is petroleum refining. It's not typically isolated as a pure component but is obtained as part of a complex mixture of hydrocarbons during the fractional distillation of crude oil. However, specific refining techniques focusing on isomerization and catalytic cracking are employed to increase the yield of branched alkanes, including TMP.
The precise synthetic route to produce pure 2,2,4,4-Tetramethylpentane involves several steps:
-
Crude Oil Distillation: Crude oil is fractionally distilled to separate hydrocarbons based on boiling points. TMP will be present in heavier fractions.
-
Isomerization: Linear alkanes in the heavier fractions are often isomerized using catalysts to generate more branched isomers, including TMP. This process is crucial because the starting materials are often linear alkanes.
-
Separation and Purification: Advanced separation techniques such as chromatography are used to isolate TMP from the complex mixture of hydrocarbons obtained after isomerization.
Applications of 2,2,4,4-Tetramethylpentane: Fuel and Beyond
The most prominent application of 2,2,4,4-Tetramethylpentane lies in its use as a high-octane fuel component. Its high octane rating makes it an essential ingredient in gasoline blends, particularly those designed for high-performance engines. The addition of TMP improves fuel efficiency and reduces engine knocking.
Beyond its application as a fuel additive, 2,2,4,4-Tetramethylpentane finds limited use in other areas:
-
Solvent: While not a common solvent, its ability to dissolve nonpolar compounds can find niche applications.
-
Chemical Intermediate: It can serve as a starting material for the synthesis of other organic molecules, although this is not a widespread application.
-
Research Purposes: TMP is frequently used in research studies exploring combustion processes, engine performance, and the properties of branched alkanes.
Frequently Asked Questions (FAQ)
Q: Is 2,2,4,4-Tetramethylpentane toxic?
A: Like most hydrocarbons, TMP is not acutely toxic at low concentrations. However, prolonged exposure or ingestion of large amounts can lead to health problems. Proper handling and safety precautions are essential when working with any hydrocarbons.
Q: What is the difference between 2,2,4,4-Tetramethylpentane and other isomers of nonane?
A: The key difference lies in the branching pattern. Different branching patterns lead to variations in boiling points, octane ratings, and other physical properties. 2,2,4,4-Tetramethylpentane's specific branching results in its exceptionally high octane rating.
Q: Can 2,2,4,4-Tetramethylpentane be synthesized in a laboratory?
A: While it's possible to synthesize TMP in a laboratory setting, the process is complex and typically not cost-effective compared to obtaining it from petroleum refining.
Q: What are the environmental impacts of 2,2,4,4-Tetramethylpentane?
A: As a component of gasoline, its combustion contributes to greenhouse gas emissions. However, its contribution to air pollution is comparable to other alkanes used in fuels.
Q: Are there any alternatives to using 2,2,4,4-Tetramethylpentane in gasoline?
A: Research continues into developing alternative fuels and fuel additives with lower environmental impacts. Biofuels and other renewable energy sources are being explored as potential alternatives.
Conclusion: A Valuable Compound with Broader Implications
2,2,4,4-Tetramethylpentane, despite its seemingly simple structure, is a compound of significant importance in the fuel industry. Its high octane rating stems directly from its highly branched structure, a testament to the remarkable impact of molecular architecture on macroscopic properties. While its direct applications outside of fuels remain limited, its contribution to high-performance engine efficiency and its role in research concerning combustion and fuel development underscore its importance in the broader context of energy and materials science. Further research into its properties and potential applications could lead to further advancements in fuel technology and other areas.
Latest Posts
Latest Posts
-
Chevy 350 Spark Plug Wires
Sep 16, 2025
-
1 Butene Vs 2 Butene
Sep 16, 2025
-
Definition Of Displacement In Chemistry
Sep 16, 2025
-
Heat Of Combustion For Butane
Sep 16, 2025
-
Solve 2x 1 X 3
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about 2 2 4 4 Tetramethylpentane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.