2 2 4 4 Tetramethylhexane
thesills
Sep 12, 2025 · 7 min read
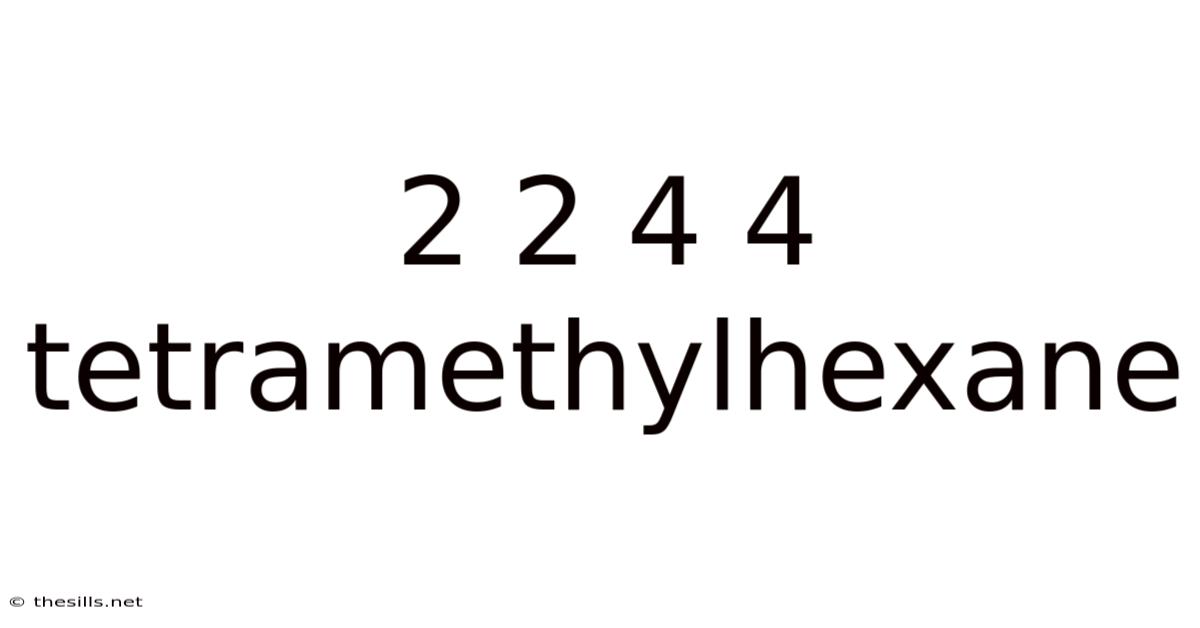
Table of Contents
Decoding 2,2,4,4-Tetramethylhexane: A Deep Dive into its Structure, Properties, and Significance
2,2,4,4-Tetramethylhexane, often shortened to TMH, is a branched-chain alkane with a fascinating structure and unique properties. Understanding its characteristics requires delving into the world of organic chemistry, exploring its isomerism, synthesis, reactivity, and potential applications. This comprehensive guide will explore these aspects, providing a detailed understanding of this intriguing molecule. This article will serve as a valuable resource for students, researchers, and anyone interested in the intricacies of organic chemistry.
Introduction to Alkanes and Branched-Chain Structures
Before diving into the specifics of 2,2,4,4-tetramethylhexane, let's establish a foundational understanding of alkanes. Alkanes are saturated hydrocarbons, meaning they consist solely of carbon and hydrogen atoms connected by single bonds. They follow the general formula C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. While simpler alkanes like methane (CH₄) and ethane (C₂H₆) exist as straight chains, as the number of carbons increases, the possibility of branching emerges. Branched-chain alkanes, like TMH, exhibit different structural arrangements of their carbon atoms, leading to variations in their physical and chemical properties. The branching significantly influences properties such as boiling point, melting point, and reactivity.
Understanding the Structure of 2,2,4,4-Tetramethylhexane
The name "2,2,4,4-tetramethylhexane" itself provides a roadmap to its structure. Let's break it down:
- Hexane: This indicates a six-carbon chain as the parent alkane.
- Tetramethyl: This signifies the presence of four methyl groups (–CH₃).
- 2,2,4,4: These numbers denote the positions of the methyl groups on the hexane chain. Two methyl groups are attached to the second carbon atom, and another two are attached to the fourth carbon atom.
This arrangement results in a highly symmetrical, compact molecule. Unlike a straight-chain hexane, TMH's branching significantly reduces its surface area and intermolecular forces. Visualizing this structure using a molecular model kit or a 3D molecular visualization program is extremely helpful for understanding its unique spatial configuration. The compact nature is crucial in determining its physical and chemical characteristics.
Isomerism and Constitutional Isomers of Hexane
It's important to understand that 2,2,4,4-tetramethylhexane is just one of many possible isomers of hexane. Isomers are molecules with the same molecular formula but different structural arrangements. Constitutional isomers, also known as structural isomers, differ in the connectivity of their atoms. Hexane itself has five constitutional isomers, each with its own unique properties. TMH stands out among these isomers due to its high degree of symmetry and its specific arrangement of methyl groups. Comparing TMH with its isomers highlights the profound impact of branching on the molecule's characteristics. Understanding isomerism is crucial in organic chemistry, as it significantly influences the properties and reactivity of molecules. The systematic naming of isomers, using IUPAC nomenclature, ensures clarity and consistency in communication within the scientific community.
Physical Properties of 2,2,4,4-Tetramethylhexane
Several physical properties of TMH are significantly affected by its highly branched structure:
-
Boiling Point: Due to its reduced surface area and weaker intermolecular forces compared to linear hexane, TMH has a lower boiling point. The compact structure reduces the points of contact between molecules, hindering strong interactions.
-
Melting Point: Similarly, its melting point is also affected by its structure. The symmetrical and compact arrangement influences the efficiency of packing in the solid state, contributing to the melting point.
-
Density: TMH's density is relatively lower than linear hexane due to its branched structure.
-
Solubility: Like most alkanes, TMH is nonpolar and therefore insoluble in water but soluble in many organic solvents.
These physical properties are crucial for understanding its potential applications and handling. The lower boiling point, for instance, means it requires less energy to vaporize, while its solubility characteristics dictate its compatibility with various solvents.
Chemical Properties and Reactivity of 2,2,4,4-Tetramethylhexane
TMH, as a saturated hydrocarbon, is relatively unreactive under normal conditions. Its primary chemical reactions involve:
-
Combustion: Like all alkanes, TMH undergoes combustion in the presence of oxygen, producing carbon dioxide, water, and heat. This reaction is exothermic and is the basis for TMH's use as a fuel (though less common than other alkanes).
-
Halogenation: Under specific conditions, TMH can undergo halogenation reactions, where hydrogen atoms are replaced by halogens (like chlorine or bromine). However, due to the steric hindrance caused by the methyl groups, the reaction might be slower or less selective than with linear alkanes.
-
Isomerization: While less likely spontaneously, under specific catalytic conditions, TMH might undergo isomerization to form other isomers of hexane.
These reactions are important in understanding TMH's chemical behavior and potential applications. The relative inertness under normal conditions contributes to its stability, while the possibility of controlled reactions, such as halogenation, provides avenues for chemical modifications.
Synthesis of 2,2,4,4-Tetramethylhexane
Synthesizing 2,2,4,4-tetramethylhexane often involves multi-step organic reactions. One potential route might start with readily available starting materials and employ several reaction steps, including alkylation and Grignard reactions to add the methyl groups to the hexane backbone. The precise synthetic routes and reaction conditions will vary depending on the available starting materials and desired efficiency. Precise control over reaction conditions is crucial to maximize yield and minimize the formation of unwanted side products. Detailed reaction schemes and mechanisms would require specialized organic chemistry knowledge and are beyond the scope of this introductory overview.
Potential Applications of 2,2,4,4-Tetramethylhexane
While not as widely used as some other alkanes, 2,2,4,4-tetramethylhexane finds niche applications, primarily due to its specific properties:
-
Solvent: Its non-polar nature and solubility in organic solvents make it a potential solvent in certain industrial processes.
-
Fuel: Though less common due to the availability of other alkanes, its combustion properties make it a potential fuel source.
-
Research purposes: Its unique structure makes it a valuable tool for studying the effects of branching on the physical and chemical properties of alkanes. It serves as a model compound in various research studies in organic chemistry.
-
Calibration standards: Its well-defined structure and easily measurable properties might make it suitable for use as a calibration standard in analytical chemistry techniques such as gas chromatography.
Frequently Asked Questions (FAQ)
Q: What is the IUPAC name of 2,2,4,4-tetramethylhexane?
A: 2,2,4,4-Tetramethylhexane is already the correct and accepted IUPAC name.
Q: Is 2,2,4,4-tetramethylhexane a liquid or a solid at room temperature?
A: It is typically a liquid at room temperature.
Q: How is 2,2,4,4-tetramethylhexane different from n-hexane?
A: n-hexane (normal hexane) is a straight-chain alkane, while 2,2,4,4-tetramethylhexane is a highly branched isomer. This branching significantly affects their boiling points, melting points, densities, and reactivities.
Q: What are the main hazards associated with handling 2,2,4,4-tetramethylhexane?
A: As with many organic solvents, precautions should be taken. It is flammable and should be handled away from open flames or ignition sources. Appropriate personal protective equipment (PPE) should be used. Appropriate ventilation should be ensured to minimize the risk of inhalation. Always refer to safety data sheets (SDS) for detailed hazard information.
Q: Are there any environmental concerns associated with 2,2,4,4-tetramethylhexane?
A: While not inherently toxic, its release into the environment should be minimized to prevent potential pollution. Its combustion contributes to greenhouse gas emissions.
Conclusion
2,2,4,4-Tetramethylhexane provides a compelling case study for understanding the impact of molecular structure on physical and chemical properties. Its highly branched structure distinguishes it from linear alkanes, affecting its boiling point, melting point, and reactivity. While not a widely used compound in large-scale applications, its unique characteristics make it valuable in research, as a solvent in specialized applications, and potentially in niche applications as a fuel or calibration standard. Further research into its properties and potential applications may reveal additional uses for this fascinating molecule. This detailed exploration offers a solid foundation for understanding this fascinating molecule and its place within the broader context of organic chemistry.
Latest Posts
Latest Posts
-
What Is A Consecutive Angle
Sep 12, 2025
-
What Is 30 Of 36
Sep 12, 2025
-
Potential And Kinetic Energy Diagrams
Sep 12, 2025
-
Conformational Isomers Vs Constitutional Isomers
Sep 12, 2025
-
Convert 86 F To C
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about 2 2 4 4 Tetramethylhexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.