Second Order Rate Constant Units
thesills
Sep 19, 2025 · 8 min read
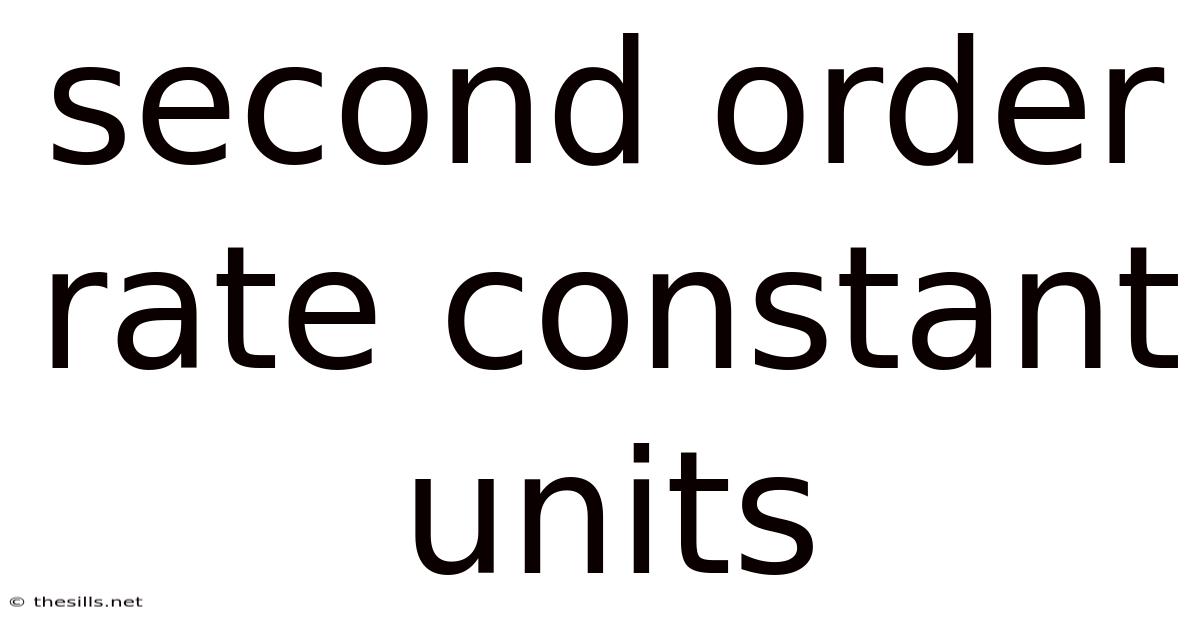
Table of Contents
Decoding the Units of Second-Order Rate Constants: A Comprehensive Guide
Understanding the units of a second-order rate constant is crucial for anyone working with chemical kinetics. This seemingly simple concept often causes confusion, particularly when dealing with different concentration units and reaction orders. This article provides a comprehensive explanation of second-order rate constants, detailing their units, how they are derived, and addressing common misconceptions. We'll explore the underlying principles, provide practical examples, and delve into the nuances of interpreting these units in various contexts. By the end, you’ll have a solid grasp of this fundamental aspect of chemical kinetics.
Understanding Reaction Order and Rate Constants
Before diving into the specifics of second-order rate constants, let's briefly review reaction order and rate constants in general. The rate law of a reaction describes the relationship between the reaction rate and the concentrations of the reactants. The overall reaction order is the sum of the exponents in the rate law, which represent the order with respect to each reactant. For example, consider a general reaction:
aA + bB → Products
The rate law might be expressed as:
Rate = k[A]<sup>x</sup>[B]<sup>y</sup>
where:
- k is the rate constant, a proportionality constant that reflects the intrinsic rate of the reaction.
- [A] and [B] are the molar concentrations of reactants A and B.
- x and y are the reaction orders with respect to A and B, respectively. These are usually integers (0, 1, 2, etc.), but can sometimes be fractional or negative.
- The overall reaction order is x + y.
Second-Order Reactions: A Closer Look
A second-order reaction is one where the overall reaction order is two. This can arise in two primary ways:
-
Second-order with respect to a single reactant: The rate law is of the form: Rate = k[A]<sup>2</sup>. This implies that the reaction rate is proportional to the square of the concentration of reactant A. Doubling the concentration of A would quadruple the reaction rate.
-
First-order with respect to two different reactants: The rate law is of the form: Rate = k[A][B]. Here, the reaction rate is proportional to the product of the concentrations of both reactants A and B. Doubling the concentration of either A or B would double the reaction rate.
Deriving the Units of the Second-Order Rate Constant
The units of the rate constant, k, depend on the overall order of the reaction. For a second-order reaction, we can derive the units by examining the rate law and ensuring dimensional consistency.
Case 1: Second-order with respect to a single reactant (Rate = k[A]<sup>2</sup>)
- Rate: The rate of a reaction has units of concentration per time (e.g., mol L<sup>-1</sup> s<sup>-1</sup>, M s<sup>-1</sup>).
- [A]<sup>2</sup>: This has units of (mol L<sup>-1</sup>)<sup>2</sup> = mol<sup>2</sup> L<sup>-2</sup>.
To ensure dimensional consistency, the units of k must satisfy:
mol L<sup>-1</sup> s<sup>-1</sup> = k × mol<sup>2</sup> L<sup>-2</sup>
Solving for k, we get:
k = (mol L<sup>-1</sup> s<sup>-1</sup>) / (mol<sup>2</sup> L<sup>-2</sup>) = L mol<sup>-1</sup> s<sup>-1</sup>
Therefore, for a second-order reaction with respect to a single reactant, the units of the rate constant are L mol<sup>-1</sup> s<sup>-1</sup> (or M<sup>-1</sup>s<sup>-1</sup>, where M represents molarity).
Case 2: First-order with respect to two different reactants (Rate = k[A][B])
- Rate: Units of mol L<sup>-1</sup> s<sup>-1</sup> (or M s<sup>-1</sup>).
- [A][B]: Units of (mol L<sup>-1</sup>)(mol L<sup>-1</sup>) = mol<sup>2</sup> L<sup>-2</sup>.
Again, ensuring dimensional consistency:
mol L<sup>-1</sup> s<sup>-1</sup> = k × mol<sup>2</sup> L<sup>-2</sup>
Solving for k, we obtain the same units as before:
k = L mol<sup>-1</sup> s<sup>-1</sup> (or M<sup>-1</sup>s<sup>-1</sup>)
Interestingly, despite the different forms of the rate law, the units of the second-order rate constant remain the same in both cases. This is because the overall reaction order is two in both scenarios.
Common Units and Their Conversions
While L mol<sup>-1</sup> s<sup>-1</sup> (or its equivalent M<sup>-1</sup>s<sup>-1</sup>) is the most commonly encountered unit, other units might be used depending on the context. For example, if the concentration is expressed in atmospheres (atm) for gaseous reactions, the units of k could be atm<sup>-1</sup> s<sup>-1</sup>. It's important to be consistent with units throughout your calculations. You may need to convert between different units of concentration or time. Standard unit conversion techniques apply here. For example, converting from L mol<sup>-1</sup> min<sup>-1</sup> to L mol<sup>-1</sup> s<sup>-1</sup> would involve multiplying by the conversion factor (1 min/60 s).
Practical Examples and Applications
Let's illustrate with a couple of examples:
Example 1: The saponification reaction of ethyl acetate with sodium hydroxide is a second-order reaction. Its rate law is:
Rate = k[CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub>][NaOH]
If the rate is determined to be 0.05 M s<sup>-1</sup> when the concentrations of ethyl acetate and NaOH are both 0.1 M, then the rate constant k can be calculated:
0.05 M s<sup>-1</sup> = k × (0.1 M) × (0.1 M)
k = 50 M<sup>-1</sup> s<sup>-1</sup> (or 50 L mol<sup>-1</sup> s<sup>-1</sup>)
Example 2: The decomposition of nitrogen dioxide (2NO<sub>2</sub> → 2NO + O<sub>2</sub>) is a second-order reaction with respect to NO<sub>2</sub>. Suppose the rate constant is determined experimentally to be 1.1 × 10<sup>-2</sup> L mol<sup>-1</sup> s<sup>-1</sup> at a particular temperature. This information is crucial in predicting the reaction rate at various concentrations of NO<sub>2</sub>.
Beyond the Basics: Temperature Dependence and Activation Energy
The rate constant, k, is temperature-dependent. The Arrhenius equation describes this relationship:
k = Ae<sup>-Ea/RT</sup>
where:
- A is the pre-exponential factor (frequency factor).
- Ea is the activation energy.
- R is the gas constant.
- T is the absolute temperature.
While the units of k are affected by the reaction order, the Arrhenius equation remains valid regardless of the reaction order. The activation energy (Ea), usually expressed in kJ/mol, represents the minimum energy required for the reaction to occur.
Frequently Asked Questions (FAQ)
Q: What if the reaction is not exactly second-order?
A: In reality, many reactions exhibit complex kinetics that deviate from simple second-order behavior. For instance, the reaction might show a second-order dependence at low concentrations but shift to a different order at higher concentrations. In such cases, the rate constant would not strictly follow the units derived for a simple second-order reaction. Analyzing the reaction mechanism and experimental data carefully is vital.
Q: Can the units of k ever be dimensionless?
A: Yes, in the unusual case of a zero-order reaction, the rate is independent of reactant concentration (Rate = k), so the units of k become the same as the units of rate (e.g., mol L<sup>-1</sup> s<sup>-1</sup>). However, a true zero-order reaction is rare and often represents a situation where another factor (e.g., catalyst concentration or light intensity) is limiting the reaction rate.
Q: How do I handle units when performing calculations involving the rate constant?
A: Always pay careful attention to units. Make sure the units of all quantities in your calculations (rate, concentrations, and the rate constant) are consistent. If necessary, convert units to a common system before proceeding with your calculations. This consistent handling of units will prevent errors and ensure the accuracy of your results.
Q: Are there any online tools or resources to help me understand and calculate rate constants?
A: While this article focuses on the theoretical understanding, many online calculators and resources can aid in calculating rate constants based on experimental data. These typically require you to input experimental rate and concentration data.
Conclusion
Understanding the units of second-order rate constants is foundational to mastering chemical kinetics. By grasping the derivation of these units and appreciating their dependence on the reaction order and concentration units, you can accurately interpret experimental data, predict reaction rates, and effectively apply the principles of chemical kinetics to a wide range of applications. Remember to always pay close attention to units throughout your calculations to avoid errors. Further exploration into reaction mechanisms, temperature dependence, and more complex reaction orders will deepen your understanding even further.
Latest Posts
Related Post
Thank you for visiting our website which covers about Second Order Rate Constant Units . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.