Ph Of 0.1 M Hcl
thesills
Sep 16, 2025 · 6 min read
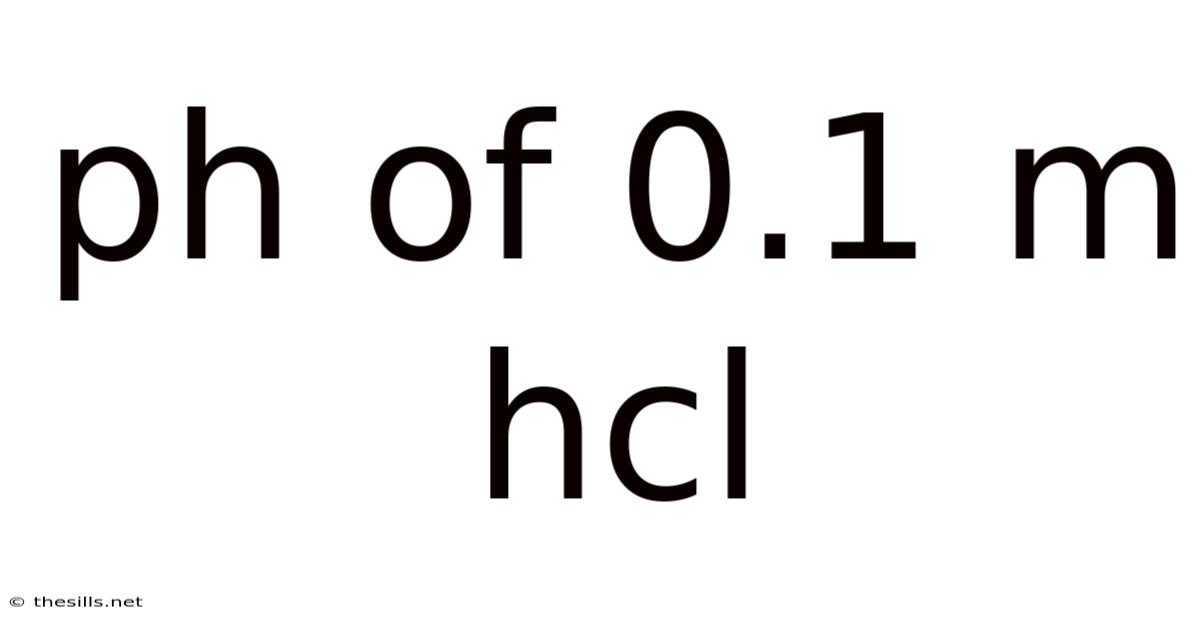
Table of Contents
Understanding the pH of 0.1 M HCl: A Deep Dive into Acid-Base Chemistry
The pH of a solution is a crucial parameter in chemistry, indicating its acidity or alkalinity. This article delves into the calculation and implications of the pH of a 0.1 M hydrochloric acid (HCl) solution. We'll explore the underlying principles of acid-base chemistry, the definition of pH, and the steps involved in determining the pH of this common strong acid. Understanding this seemingly simple calculation provides a strong foundation for more complex acid-base chemistry problems.
Introduction to pH and the pH Scale
The pH scale is a logarithmic scale used to specify the acidity or basicity (alkalinity) of an aqueous solution. It ranges from 0 to 14, with 7 representing neutrality. Solutions with a pH less than 7 are acidic, while those with a pH greater than 7 are basic or alkaline. The scale is based on the concentration of hydrogen ions (H⁺) in the solution. The pH is defined as the negative logarithm (base 10) of the hydrogen ion concentration:
pH = -log₁₀[H⁺]
Where [H⁺] represents the concentration of hydrogen ions in moles per liter (Molarity). A lower pH indicates a higher concentration of H⁺ ions and therefore a stronger acid. Conversely, a higher pH indicates a lower concentration of H⁺ ions and a weaker acid or a stronger base.
Understanding Strong Acids and HCl
Hydrochloric acid (HCl) is a strong acid. This means that it completely dissociates (ionizes) in water, meaning every molecule of HCl breaks apart into its constituent ions: a hydrogen ion (H⁺) and a chloride ion (Cl⁻). This complete dissociation is crucial for calculating the pH of HCl solutions. The reaction can be represented as:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The complete dissociation of HCl simplifies the pH calculation because the concentration of H⁺ ions is directly equal to the initial concentration of HCl.
Calculating the pH of 0.1 M HCl
Since HCl is a strong acid and completely dissociates, the concentration of H⁺ ions in a 0.1 M HCl solution is also 0.1 M. We can now use the pH formula:
pH = -log₁₀[H⁺] = -log₁₀(0.1)
The logarithm of 0.1 (or 10⁻¹) is -1. Therefore:
pH = -(-1) = 1
The pH of a 0.1 M HCl solution is 1. This indicates a highly acidic solution.
The Significance of the pH Value: Implications of High Acidity
A pH of 1 signifies a very strong acidic solution. Solutions with this level of acidity can be highly corrosive and dangerous. It's important to handle 0.1 M HCl with appropriate safety precautions, including:
- Eye protection: Always wear safety goggles or a face shield.
- Gloves: Wear chemical-resistant gloves.
- Lab coat: Protect your clothing with a lab coat.
- Ventilation: Work in a well-ventilated area or under a fume hood to avoid inhaling the fumes.
- Neutralization procedures: Know how to neutralize spills safely using a suitable base.
The corrosive nature of 0.1 M HCl stems from the high concentration of H⁺ ions. These ions react readily with many substances, breaking down their structures. This is why it's crucial to handle strong acids carefully and follow proper laboratory safety procedures.
Beyond the Simple Calculation: Factors Affecting pH
While the calculation for 0.1 M HCl is straightforward due to its complete dissociation, there are factors that can affect the pH in more complex scenarios:
-
Temperature: Temperature affects the dissociation constant of weak acids. While the impact on strong acids like HCl is minimal at common lab temperatures, significant temperature changes could subtly affect the pH.
-
Ionic Strength: The presence of other ions in solution can affect the activity of H⁺ ions, which in turn influences the measured pH. This effect is described by the activity coefficient. In dilute solutions like 0.1 M HCl, the ionic strength effect is relatively small, but it becomes more significant at higher concentrations.
-
Activity vs. Concentration: The pH calculation above uses the concentration of H⁺ ions. However, a more accurate representation involves using the activity of H⁺ ions, which accounts for interionic interactions. In dilute solutions, the activity is approximately equal to the concentration, justifying our simplification.
-
Dilution: Diluting the 0.1 M HCl solution will increase the pH. As the concentration of H⁺ ions decreases, the pH will rise closer to 7 (neutral).
Weak Acids and the Difference in pH Calculation
The calculation of pH for a weak acid is considerably more complex than that for a strong acid. Weak acids do not completely dissociate in water. Instead, an equilibrium is established between the undissociated acid and its ions. To calculate the pH of a weak acid solution, we need to use the acid dissociation constant (Ka) and the equilibrium expression. The calculation often involves the quadratic formula or approximations, making it significantly more challenging than the calculation for a strong acid like HCl.
Frequently Asked Questions (FAQs)
Q: What happens if I add water to 0.1 M HCl?
A: Adding water dilutes the solution, decreasing the concentration of H⁺ ions and thus increasing the pH. The pH will move closer to 7, but it will still remain acidic.
Q: Can I calculate the pH of a mixture of 0.1 M HCl and another acid?
A: If the other acid is also a strong acid, you can simply add the concentrations of H⁺ ions from both acids to find the total [H⁺] and then calculate the pH. If the other acid is a weak acid, the calculation becomes more complex and requires consideration of the weak acid's equilibrium.
Q: What are some practical applications where understanding the pH of 0.1 M HCl is important?
A: Understanding the pH of 0.1 M HCl is crucial in various applications, including:
- Titrations: In acid-base titrations, 0.1 M HCl is often used as a standard solution to determine the concentration of unknown bases.
- Chemical synthesis: Many chemical reactions are pH-dependent, and 0.1 M HCl can be used to control the pH of a reaction mixture.
- Industrial processes: 0.1 M HCl is used in various industrial processes, including metal cleaning and pickling.
- Analytical chemistry: It's used in various analytical techniques to adjust pH and create specific chemical environments.
Q: What are the safety precautions when working with 0.1 M HCl?
A: Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat. Work in a well-ventilated area or under a fume hood. Familiarize yourself with proper spill procedures and neutralization techniques.
Conclusion
The pH of a 0.1 M HCl solution is a fundamental concept in acid-base chemistry. Understanding its calculation (pH = 1) highlights the significance of strong acid dissociation and the implications of high acidity. This knowledge forms a cornerstone for further exploration of more complex acid-base systems and emphasizes the importance of safety precautions when handling strong acids in any laboratory or industrial setting. While the calculation for 0.1 M HCl is relatively straightforward, remember to consider factors such as temperature, ionic strength, and the distinction between concentration and activity for a more nuanced understanding of pH in various chemical environments. The seemingly simple calculation of the pH of 0.1 M HCl opens doors to a deeper understanding of the intricate world of acid-base chemistry and its applications.
Latest Posts
Latest Posts
-
Vant Hoff Factor Of Urea
Sep 16, 2025
-
Molecular Mass Of Zinc Carbonate
Sep 16, 2025
-
Equivalent Fraction Of 8 12
Sep 16, 2025
-
Integral Of Square Root X
Sep 16, 2025
-
Magnesium Oxide Reacting With Water
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about Ph Of 0.1 M Hcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.