Ph Of 0.01 M Naoh
thesills
Sep 13, 2025 · 5 min read
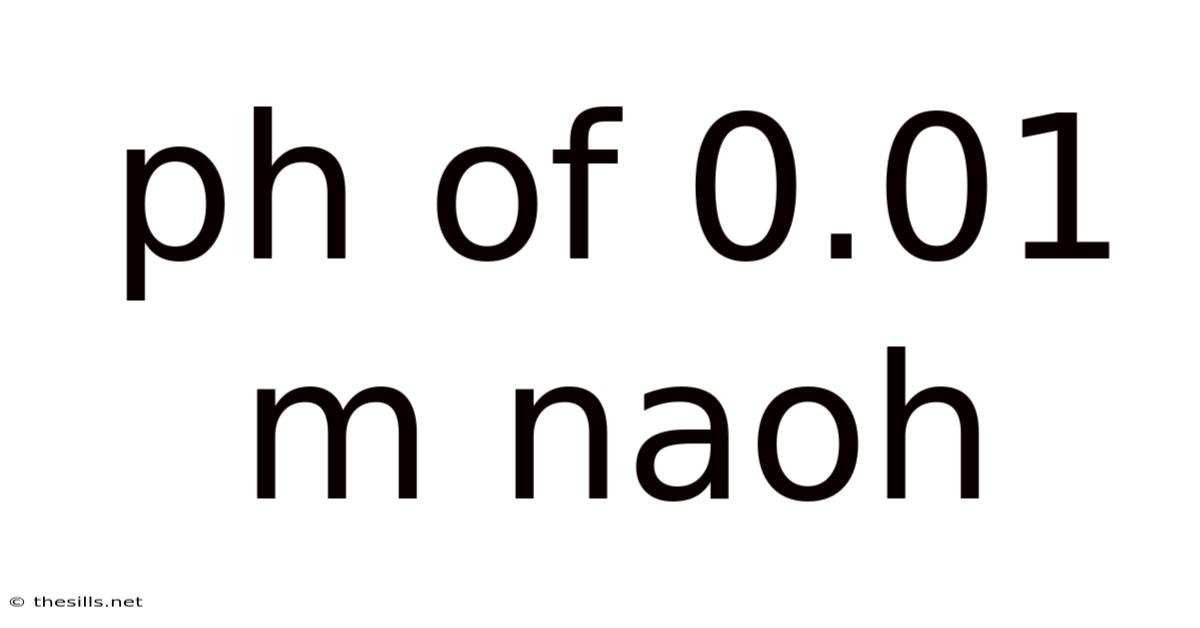
Table of Contents
Calculating and Understanding the pH of 0.01 M NaOH
Determining the pH of a 0.01 M solution of sodium hydroxide (NaOH) is a fundamental exercise in chemistry, crucial for understanding acid-base concepts and equilibrium calculations. This article will guide you through the process, explaining the underlying principles and providing a detailed step-by-step approach. We will also delve into the implications of this calculation and explore related concepts, ensuring a comprehensive understanding.
Introduction: Understanding pH and pOH
Before diving into the calculation, let's refresh our understanding of key concepts. pH is a measure of the hydrogen ion (H⁺) concentration in a solution, expressed on a logarithmic scale. The formula for pH is:
pH = -log₁₀[H⁺]
where [H⁺] represents the molar concentration of hydrogen ions. A lower pH indicates a higher concentration of H⁺ and thus a more acidic solution. Conversely, a higher pH indicates a lower concentration of H⁺ and a more alkaline (basic) solution.
pOH, similarly, represents the hydroxide ion (OH⁻) concentration:
pOH = -log₁₀[OH⁻]
The relationship between pH and pOH in aqueous solutions at 25°C is crucial:
pH + pOH = 14
This equation stems from the ion product constant of water (Kw), which is 1.0 x 10⁻¹⁴ at 25°C. Kw = [H⁺][OH⁻] = 1.0 x 10⁻¹⁴.
NaOH: A Strong Base
Sodium hydroxide (NaOH) is a strong base, meaning it completely dissociates in water. This complete dissociation is key to simplifying our pH calculation. When NaOH dissolves in water, it completely ionizes into sodium ions (Na⁺) and hydroxide ions (OH⁻):
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
This means that the concentration of OH⁻ ions in a 0.01 M NaOH solution is equal to 0.01 M.
Step-by-Step Calculation of the pH of 0.01 M NaOH
-
Determine the concentration of OH⁻: As NaOH is a strong base and completely dissociates, the concentration of OH⁻ ions is equal to the concentration of NaOH. Therefore, [OH⁻] = 0.01 M = 1.0 x 10⁻² M.
-
Calculate the pOH: Using the pOH formula:
pOH = -log₁₀[OH⁻] = -log₁₀(1.0 x 10⁻²) = 2
-
Calculate the pH: Using the relationship between pH and pOH:
pH + pOH = 14 pH = 14 - pOH = 14 - 2 = 12
Therefore, the pH of a 0.01 M NaOH solution is 12. This indicates a highly alkaline solution.
Understanding the Implications: The Significance of pH 12
A pH of 12 signifies a strongly alkaline solution. Such solutions can be corrosive and are capable of causing chemical burns. They are commonly found in various industrial processes, drain cleaners, and some soap solutions. Understanding the pH is crucial for safety and handling procedures. It's important to always use appropriate personal protective equipment (PPE) when working with such solutions.
Factors Affecting pH: Temperature and Ionic Strength
While the calculation above assumes a temperature of 25°C, the pH of a solution can be affected by temperature changes. The ion product constant of water (Kw) is temperature-dependent; as temperature increases, Kw increases, and this affects the relationship between pH and pOH. At higher temperatures, the pH of a 0.01 M NaOH solution will be slightly lower than 12.
Ionic strength, representing the total concentration of ions in a solution, can also affect pH measurements. High ionic strength can influence the activity of ions, leading to deviations from the calculated pH. This is usually accounted for by using activity coefficients, which are beyond the scope of this basic calculation but are important for more precise measurements.
Advanced Considerations: Activity Coefficients and Debye-Hückel Theory
The calculation we performed is based on the assumption that the activity of the hydroxide ions is equal to their concentration. This is a simplification, and in reality, the activity of ions is affected by the presence of other ions in the solution. Activity coefficients correct for this deviation. The Debye-Hückel theory provides a way to estimate activity coefficients, offering a more accurate representation of the system’s behavior.
Practical Applications and Examples
The concept of calculating pH is not limited to simple solutions like 0.01 M NaOH. It has widespread applications in various fields:
- Environmental Monitoring: Determining the pH of water samples is crucial for assessing water quality and protecting aquatic life. Acid rain, for example, significantly lowers the pH of water bodies.
- Analytical Chemistry: pH measurements are essential in titrations, where the pH change is used to determine the concentration of unknown solutions.
- Industrial Processes: pH control is vital in many industrial processes, including manufacturing, food processing, and pharmaceuticals, to ensure optimal reaction conditions and product quality.
- Medicine and Biology: pH plays a critical role in biological systems. The pH of blood, for instance, is tightly regulated within a narrow range (around 7.4) to maintain proper physiological function.
Frequently Asked Questions (FAQ)
-
Q: What happens if the concentration of NaOH is increased?
- A: Increasing the concentration of NaOH will increase the concentration of OH⁻ ions, leading to a higher pOH and a correspondingly higher pH (closer to 14).
-
Q: What happens if the temperature is increased?
- A: Increasing the temperature increases Kw. This will result in a slightly lower pH than what was calculated at 25°C.
-
Q: Can we use this calculation for weak bases?
- A: No. This calculation only applies to strong bases because they completely dissociate. Calculations for weak bases are more complex and involve equilibrium constants (Kb).
-
Q: What are some common strong bases other than NaOH?
- A: Other common strong bases include KOH (potassium hydroxide), Ba(OH)₂ (barium hydroxide), and Ca(OH)₂ (calcium hydroxide).
-
Q: How is pH measured in a laboratory setting?
- A: pH is typically measured using a pH meter, a device that uses a special electrode sensitive to hydrogen ion concentration.
Conclusion
Calculating the pH of a 0.01 M NaOH solution is a straightforward yet crucial exercise in understanding acid-base chemistry. The complete dissociation of NaOH simplifies the calculation, allowing us to directly determine the pOH and subsequently the pH. However, it’s important to remember that this calculation is based on ideal conditions and may deviate slightly in real-world scenarios due to factors like temperature and ionic strength. Understanding these limitations and the broader applications of pH calculations is essential for anyone working with chemical solutions. This knowledge forms a fundamental building block for more advanced concepts in chemistry and related fields. The significance of pH extends far beyond theoretical calculations, playing a vital role in various aspects of science, industry, and environmental monitoring.
Latest Posts
Latest Posts
-
Are Amoebas Prokaryotic Or Eukaryotic
Sep 13, 2025
-
Silverfish Army Ants Info On Relationship
Sep 13, 2025
-
Magnetic Flux Through The Loop
Sep 13, 2025
-
14k Gold Jewelry Making Kit
Sep 13, 2025
-
As Disposable Income Increases Consumption
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about Ph Of 0.01 M Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.