Na H2o Naoh H2 Balanced
thesills
Sep 16, 2025 · 7 min read
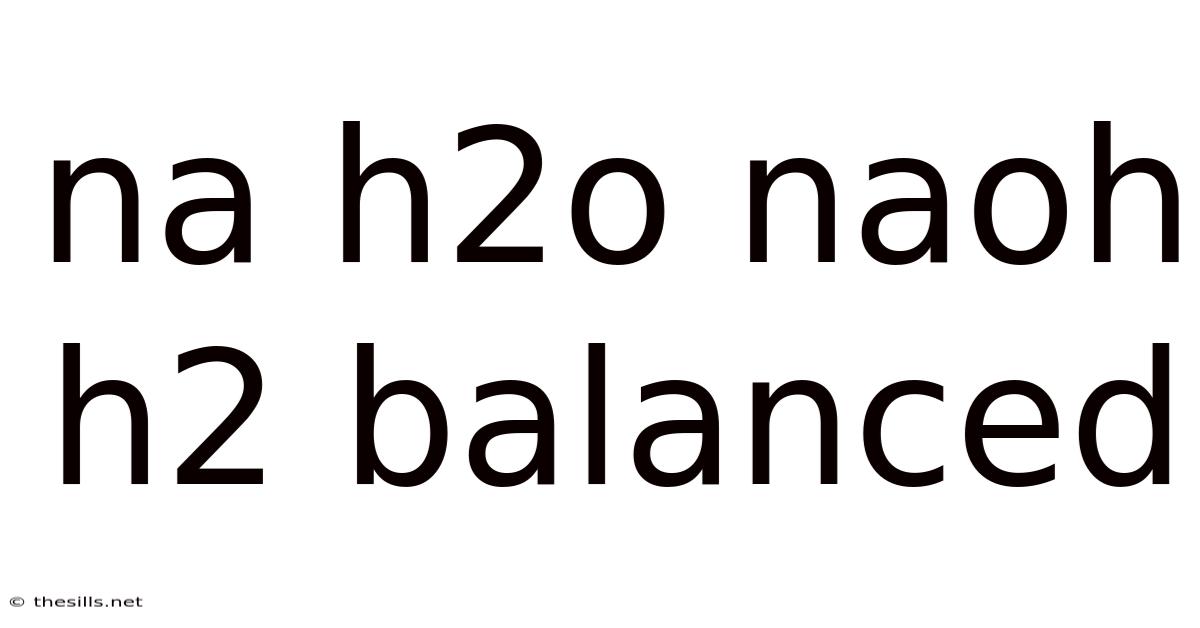
Table of Contents
Understanding the Balanced Chemical Equation: Na + H₂O → NaOH + H₂
The reaction between sodium (Na) and water (H₂O) is a classic example of a highly exothermic single displacement reaction, producing sodium hydroxide (NaOH) and hydrogen gas (H₂). This seemingly simple equation, Na + H₂O → NaOH + H₂, hides a wealth of chemical principles and interesting observations. Understanding this reaction requires examining the balancing process, the underlying chemical mechanisms, and the safety precautions involved. This article will delve into each aspect, providing a comprehensive understanding of the reaction and its implications.
Introduction: A Vigorous Reaction
The reaction between sodium metal and water is highly reactive and exothermic, meaning it releases a significant amount of heat. The reaction is so vigorous that the generated hydrogen gas often ignites spontaneously, producing a bright orange flame. This dramatic demonstration highlights the importance of handling sodium metal with extreme caution. The balanced chemical equation accurately reflects the stoichiometry of the reaction, ensuring that the number of atoms of each element is the same on both the reactant and product sides. Understanding how to balance this equation is crucial for comprehending the reaction's quantitative aspects.
Balancing the Chemical Equation: A Step-by-Step Approach
The unbalanced equation, Na + H₂O → NaOH + H₂, is not stoichiometrically correct. Balancing a chemical equation involves adjusting the coefficients (the numbers in front of the chemical formulas) to ensure that the number of atoms of each element is equal on both sides of the equation. Here's a step-by-step approach to balancing this equation:
-
Identify the elements: We have sodium (Na), hydrogen (H), and oxygen (O).
-
Count the atoms: On the reactant side, we have 1 Na, 2 H, and 1 O. On the product side, we have 1 Na, 3 H, and 1 O.
-
Balance the elements: We can start by balancing the hydrogen atoms. There are two hydrogen atoms on the reactant side and three on the product side. To balance this, we can put a coefficient of 2 in front of H₂O and a coefficient of 2 in front of NaOH:
Na + 2H₂O → 2NaOH + H₂
-
Recount the atoms: Now, we have 1 Na, 4 H, and 2 O on the reactant side, and 2 Na, 2H + 2H = 4 H, and 2 O on the product side.
-
Final Balance: The hydrogen and oxygen atoms are now balanced, but the sodium is not. We add a coefficient of 2 in front of Na on the reactant side to achieve a balanced equation:
2Na + 2H₂O → 2NaOH + H₂
This balanced equation shows that two moles of sodium react with two moles of water to produce two moles of sodium hydroxide and one mole of hydrogen gas. This stoichiometric relationship is crucial for quantitative analysis of the reaction.
The Underlying Chemistry: Ionic Reactions and Oxidation-Reduction
The reaction between sodium and water is fundamentally an oxidation-reduction (redox) reaction. Sodium is a highly reactive alkali metal that readily loses one electron to achieve a stable noble gas electron configuration. Water, although relatively stable, can act as an oxidizing agent in this context.
-
Sodium's Oxidation: Sodium (Na) readily loses one electron to become a sodium ion (Na⁺):
Na → Na⁺ + e⁻
This is an oxidation process because sodium loses an electron, increasing its oxidation state from 0 to +1.
-
Water's Reduction: Water acts as an oxidizing agent, accepting electrons from sodium. One water molecule accepts an electron, undergoing reduction to form a hydroxide ion (OH⁻) and a hydrogen atom:
H₂O + e⁻ → OH⁻ + H
Since two electrons are released by two sodium atoms, two water molecules are needed to accept these electrons. The two hydrogen atoms then combine to form hydrogen gas (H₂):
2H → H₂
This overall process leads to the formation of sodium hydroxide (NaOH) and hydrogen gas (H₂), as indicated in the balanced equation. The reaction is highly exothermic because of the strong ionic bonds formed in sodium hydroxide and the release of energy during the oxidation-reduction process. The energy released is enough to ignite the hydrogen gas, causing a characteristic fiery reaction.
Safety Precautions: Handling Sodium Metal
Sodium is a highly reactive metal that reacts violently with water. Therefore, extreme caution must be exercised when handling sodium. Never handle sodium with bare hands, as it can cause severe burns. Always use appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat.
Here are some essential safety precautions:
-
Small quantities: Use only small pieces of sodium, typically no larger than a pea.
-
Controlled environment: Conduct the reaction under a fume hood to contain any hydrogen gas produced.
-
Proper disposal: Dispose of sodium hydroxide and other waste products according to established laboratory protocols.
-
Fire suppression: Have a fire extinguisher readily available in case of a fire.
Applications of the Reaction: Industrial and Laboratory Uses
The reaction between sodium and water, although seemingly simple, has several important applications:
-
Production of Sodium Hydroxide: The industrial production of sodium hydroxide (lye or caustic soda), a crucial chemical used in numerous industries, often involves variations of this reaction. While other methods are used commercially on a larger scale, this fundamental chemical principle remains relevant.
-
Hydrogen Gas Generation: This reaction can be used in the laboratory to generate small quantities of hydrogen gas, although safer and more controlled methods are generally preferred. However, the reaction's demonstration can be a powerful teaching tool for illustrating redox reactions and stoichiometry.
-
Chemical Demonstrations: The dramatic nature of the reaction makes it a popular and effective demonstration in chemistry education, highlighting the reactivity of alkali metals and the principles of oxidation-reduction.
-
Understanding Alkali Metal Reactivity: The reaction illustrates the characteristic reactivity of alkali metals and their tendency to readily lose electrons, helping to solidify understanding of periodic trends and chemical properties.
Frequently Asked Questions (FAQ)
Q: Why is the reaction so exothermic?
A: The reaction is exothermic due to the strong ionic bonds formed in sodium hydroxide (NaOH) and the relatively weak bonds broken in sodium metal and water. The energy released during the formation of these strong ionic bonds significantly exceeds the energy required to break the existing bonds, resulting in a large release of heat. The oxidation-reduction process itself also contributes significantly to the heat released.
Q: Can other alkali metals react similarly with water?
A: Yes, other alkali metals (lithium, potassium, rubidium, cesium, and francium) also react vigorously with water, although the reactivity generally increases as you go down the group. Lithium reacts less violently, while potassium, rubidium, and cesium react even more violently than sodium. Francium is extremely radioactive and not readily available for such experiments.
Q: What happens if I add a large amount of sodium to water?
A: Adding a large amount of sodium to water can result in a very violent reaction, potentially leading to an explosion due to the rapid production of hydrogen gas. This emphasizes the importance of using only small quantities of sodium in controlled laboratory settings.
Q: What are the safety hazards associated with this reaction?
A: The primary safety hazards are the potential for burns from the heat generated, the risk of explosion from the rapid production of hydrogen gas, and the corrosive nature of sodium hydroxide. Always use appropriate safety precautions, including PPE and a controlled environment, when performing this reaction.
Conclusion: A Powerful Reaction with Broad Implications
The reaction between sodium and water, represented by the balanced equation 2Na + 2H₂O → 2NaOH + H₂, is a powerful example of a redox reaction showcasing the reactivity of alkali metals and the principles of stoichiometry. Understanding the balancing of this equation, the underlying chemical mechanisms, and the associated safety precautions is crucial for anyone working with sodium metal or studying chemical reactions. While the reaction's dramatic nature makes it a compelling demonstration, it also underscores the importance of careful and safe handling of reactive chemicals in both educational and industrial settings. The knowledge gained from understanding this seemingly simple reaction extends to a deeper appreciation of fundamental chemical principles and their real-world applications.
Latest Posts
Latest Posts
-
Graph Of 1 X 1
Sep 16, 2025
-
Covalency Of Nitrogen In Hno3
Sep 16, 2025
-
Ko2 Oxidation Number Of K
Sep 16, 2025
-
How To Find Your Phenotype
Sep 16, 2025
-
Enthalpy Of Formation Of Hydrogen
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about Na H2o Naoh H2 Balanced . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.