In Of H2o To Pa
thesills
Sep 18, 2025 · 5 min read
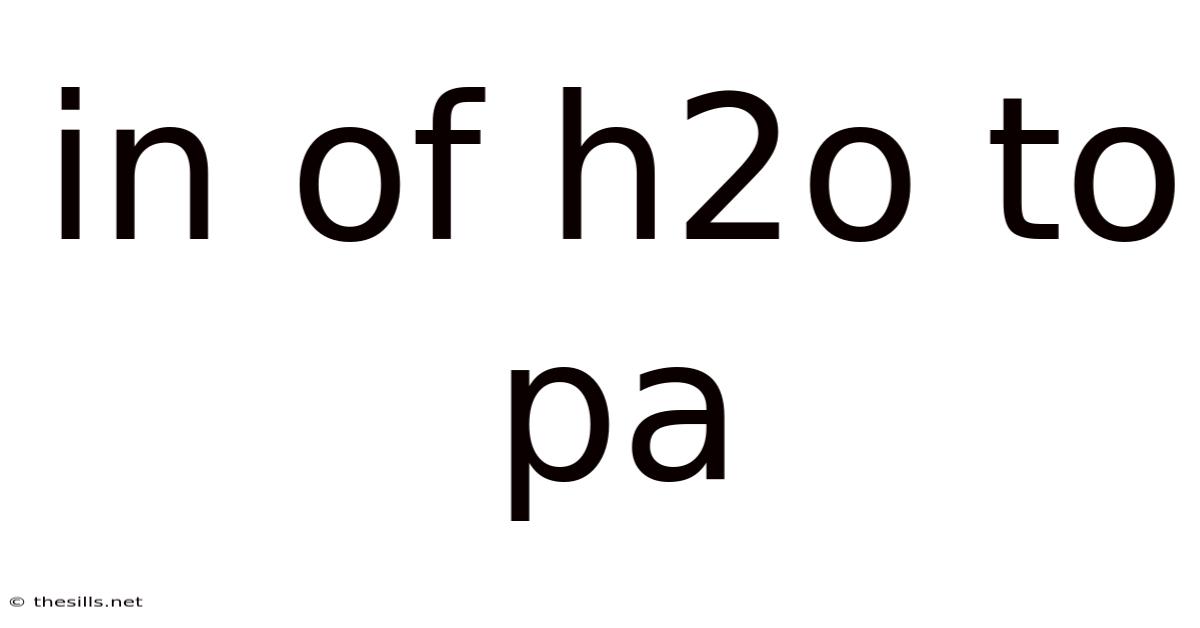
Table of Contents
Understanding the Conversion of Inches of Water (in H2O) to Pascals (Pa)
This article explores the conversion between inches of water (in H2O), a unit often used to measure low pressures, and Pascals (Pa), the standard unit of pressure in the International System of Units (SI). We will delve into the underlying physics, provide a step-by-step guide for the conversion, address common misconceptions, and answer frequently asked questions. Understanding this conversion is crucial in various fields, including meteorology, hydrology, and engineering, where pressure measurements are essential.
Introduction: Pressure and its Units
Pressure is defined as the force applied perpendicularly to a surface per unit area. Different units are used to express pressure depending on the application and the magnitude of the pressure being measured. While Pascals (Pa) are the standard SI unit, representing one Newton per square meter (N/m²), inches of water (in H2O) is a commonly used unit, particularly for measuring relatively low pressures like those found in ventilation systems or water columns. One inch of water represents the pressure exerted by a column of water one inch high. This seemingly simple unit hides a complexity linked to the density of water, temperature, and gravitational acceleration.
The Physics Behind the Conversion
The conversion from in H2O to Pa relies on understanding the relationship between pressure, height, density, and gravity. The pressure at the bottom of a column of fluid is given by the hydrostatic pressure equation:
P = ρgh
Where:
- P is the pressure (in Pascals)
- ρ (rho) is the density of the fluid (in kg/m³)
- g is the acceleration due to gravity (approximately 9.81 m/s²)
- h is the height of the fluid column (in meters)
To convert inches of water to Pascals, we need to account for the units involved. First, we must convert inches to meters and then apply the hydrostatic pressure equation using the density of water.
Step-by-Step Conversion: Inches of Water (in H2O) to Pascals (Pa)
Here's a detailed breakdown of the conversion process:
-
Convert inches to meters: There are approximately 0.0254 meters in one inch. Therefore, multiply the value in inches of water by 0.0254 to obtain the height in meters.
-
Determine the density of water: The density of water varies slightly with temperature and pressure. For most practical purposes, we can use the standard density of water at 4°C (39.2°F), which is approximately 1000 kg/m³. However, for higher accuracy, especially in critical applications, you should use the density corresponding to the specific temperature of the water.
-
Apply the hydrostatic pressure equation: Substitute the height in meters (from step 1), the density of water (from step 2), and the acceleration due to gravity (9.81 m/s²) into the hydrostatic pressure equation (P = ρgh) to calculate the pressure in Pascals.
Example:
Let's convert 1 inch of water (in H2O) to Pascals (Pa):
-
Height in meters: 1 in H2O * 0.0254 m/in = 0.0254 m
-
Density of water: We'll use the standard value of 1000 kg/m³
-
Pressure in Pascals: P = (1000 kg/m³)(9.81 m/s²)(0.0254 m) ≈ 249.08 Pa
Therefore, 1 inch of water is approximately equal to 249.08 Pascals.
Factors Affecting Accuracy: Temperature and Gravity
While the conversion process outlined above provides a good approximation, several factors can influence its accuracy:
-
Temperature: The density of water changes with temperature. Colder water is denser than warmer water. This variation in density directly affects the pressure calculated. For precise conversions, the density of water at the specific temperature should be used. Consult a density table for water to find the correct value.
-
Gravity: The acceleration due to gravity (g) is not constant across the Earth's surface. It varies slightly with altitude and latitude. While the difference is small in most cases, for high-precision measurements, the local value of g should be used in the calculation.
Common Misconceptions and Clarifications
-
Assuming constant density: It's crucial to remember that the density of water is not always 1000 kg/m³. This value is an approximation, and using a more accurate density based on the water's temperature significantly improves the accuracy of the conversion.
-
Ignoring gravity variations: While the variations in 'g' are relatively small, they can become significant in high-precision applications. Ignoring these variations can lead to errors in the calculated pressure.
-
Unit consistency: Always ensure consistent units throughout the calculation. Converting all values to SI units (meters, kilograms, seconds) before applying the hydrostatic pressure equation minimizes errors.
Frequently Asked Questions (FAQ)
-
Q: Why use inches of water instead of Pascals?
- A: Inches of water is a historically established unit, particularly in certain industries like HVAC (Heating, Ventilation, and Air Conditioning) and some older pressure gauges. It provides a readily understandable scale for relatively low pressures.
-
Q: How accurate is this conversion method?
- A: The accuracy depends on the precision of the input values (height and water density) and the consideration of factors like temperature and gravity variations. Using a standard density of water offers a reasonable approximation, but employing temperature-specific density values enhances accuracy.
-
Q: Can this conversion be applied to other liquids?
- A: Yes, but you must replace the density of water (ρ) with the density of the specific liquid you're working with. The hydrostatic pressure equation remains the same.
-
Q: What about pressures significantly higher than those measured in inches of water?
- A: For higher pressures, in H2O becomes impractical, and other pressure units like Pascals, bars, or atmospheres are more suitable.
-
Q: Are there online calculators for this conversion?
- A: Yes, many online calculators are available that perform this conversion automatically. However, understanding the underlying principles is vital for interpreting the results accurately.
Conclusion: Mastering the in H2O to Pa Conversion
Converting inches of water to Pascals requires understanding the fundamental principles of hydrostatics and paying attention to details like the density of water and the acceleration due to gravity. While a simple formula can provide a reasonable approximation, precise conversions necessitate considering temperature variations and using the appropriate density of water for the specific temperature. By grasping the underlying physics and applying the steps carefully, you can accurately convert between these two pressure units, a crucial skill in many scientific and engineering applications. Remember, accurate measurements are essential for correct interpretations and reliable results in any field relying on pressure readings. Always strive for precision and understand the limitations of your chosen method.
Latest Posts
Latest Posts
-
Resonance Structure Of Phenoxide Ion
Sep 18, 2025
-
Dropping Magnet Through Copper Tube
Sep 18, 2025
-
How Do Solar Cooker Work
Sep 18, 2025
-
Is Nh3 A Strong Base
Sep 18, 2025
-
Mark Wayne Roll Top Desk
Sep 18, 2025
Related Post
Thank you for visiting our website which covers about In Of H2o To Pa . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.