H2o Co2 H2co3 H+ Hco3
thesills
Sep 16, 2025 · 7 min read
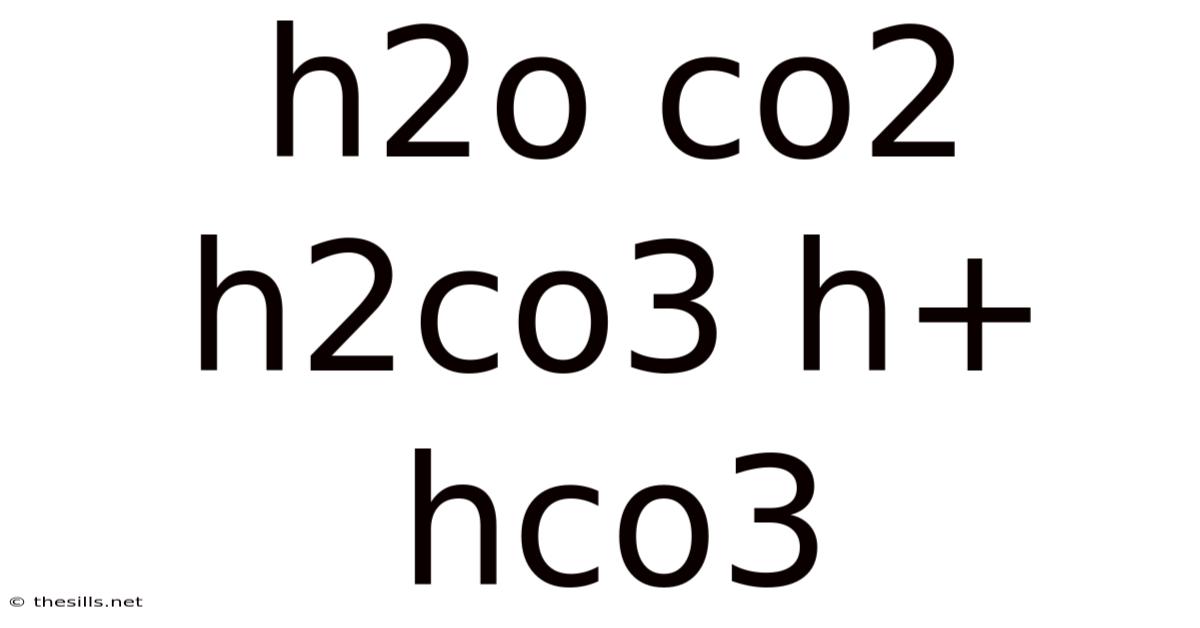
Table of Contents
Understanding the Chemistry of Carbonic Acid: H₂O, CO₂, H₂CO₃, H⁺, and HCO₃⁻
The seemingly simple chemical equation representing the formation of carbonic acid from carbon dioxide and water underpins a vast array of crucial natural processes, from ocean acidification to blood pH regulation. This article delves into the intricate relationships between H₂O (water), CO₂ (carbon dioxide), H₂CO₃ (carbonic acid), H⁺ (hydrogen ions), and HCO₃⁻ (bicarbonate ions), exploring their individual properties and their interconnected roles in various biological and environmental systems. Understanding this chemistry is key to grasping many essential concepts in environmental science, biology, and chemistry.
Introduction: A Foundation in Aqueous Chemistry
Before diving into the specifics of carbonic acid, it's vital to establish a fundamental understanding of aqueous solutions. Water (H₂O), a polar molecule, acts as a universal solvent, dissolving a wide range of substances. Its polarity allows it to interact with ionic compounds and polar molecules, leading to their dissociation or dissolution. This ability is critical to the formation and behavior of carbonic acid and its related ions.
The concept of pH, a measure of the concentration of hydrogen ions (H⁺) in a solution, is equally important. A lower pH indicates a higher concentration of H⁺, signifying a more acidic solution. Conversely, a higher pH indicates a lower concentration of H⁺, signifying a more alkaline (basic) solution. The equilibrium between H⁺, HCO₃⁻, and H₂CO₃ plays a crucial role in determining the pH of various systems, particularly natural water bodies and biological fluids.
The Formation of Carbonic Acid (H₂CO₃): A Reversible Reaction
Carbon dioxide (CO₂), a colorless and odorless gas, dissolves in water to form carbonic acid (H₂CO₃). However, this reaction is not a straightforward, complete conversion. Instead, it's an equilibrium reaction, meaning the forward and reverse reactions occur simultaneously:
CO₂(aq) + H₂O(l) ⇌ H₂CO₃(aq)
The double arrow (⇌) signifies that the reaction proceeds in both directions. Only a small fraction of dissolved CO₂ actually forms H₂CO₃; most remains as dissolved CO₂. The equilibrium constant for this reaction is relatively small, indicating that the formation of H₂CO₃ is favored only under specific conditions. Factors such as pressure and temperature influence the position of this equilibrium. Higher pressure favors the formation of H₂CO₃, while higher temperature favors the reverse reaction, releasing CO₂.
Dissociation of Carbonic Acid: The Role of H⁺ and HCO₃⁻
Carbonic acid, being a weak acid, does not fully dissociate in water. It undergoes a stepwise dissociation process:
- First Dissociation: H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
This step involves the release of a proton (H⁺) from carbonic acid, forming bicarbonate ion (HCO₃⁻). The equilibrium constant for this reaction (Kₐ₁) is relatively small, further highlighting the weak acidic nature of carbonic acid.
- Second Dissociation: HCO₃⁻(aq) ⇌ H⁺(aq) + CO₃²⁻(aq)
The bicarbonate ion can further dissociate, releasing another proton and forming carbonate ion (CO₃²⁻). The equilibrium constant for this reaction (Kₐ₂) is even smaller than Kₐ₁, indicating that the second dissociation is even less favorable than the first.
These dissociation reactions are crucial in understanding the buffering capacity of carbonic acid systems. The presence of both H₂CO₃ and HCO₃⁻ allows the system to resist changes in pH, effectively buffering against the addition of either acids or bases.
The Bicarbonate Buffer System: Maintaining pH Balance
The bicarbonate buffer system is a vital mechanism for maintaining pH homeostasis in various biological and environmental systems. In blood, for example, the equilibrium between CO₂, H₂CO₃, H⁺, and HCO₃⁻ is tightly regulated to keep the blood pH within a narrow range (approximately 7.35-7.45). This tight control is essential for optimal enzyme function and overall physiological processes. Any significant deviation from this range can lead to serious health consequences, such as acidosis or alkalosis.
The lungs and kidneys play crucial roles in regulating the bicarbonate buffer system. The lungs control the partial pressure of CO₂, which directly affects the concentration of dissolved CO₂ and consequently, the formation of H₂CO₃. The kidneys regulate the excretion of H⁺ and HCO₃⁻, further fine-tuning the pH balance.
Environmental Significance: Ocean Acidification
The interaction between CO₂, H₂O, H₂CO₃, H⁺, and HCO₃⁻ is not limited to biological systems; it also plays a significant role in shaping environmental conditions. The increasing atmospheric concentration of CO₂ due to human activities has a profound impact on the ocean's chemistry, leading to a phenomenon known as ocean acidification.
As atmospheric CO₂ dissolves in seawater, it increases the concentration of H₂CO₃, leading to a decrease in ocean pH. This increased acidity has detrimental effects on marine life, particularly organisms with calcium carbonate shells or skeletons (e.g., corals, shellfish). The increased H⁺ concentration interferes with the formation of calcium carbonate, making it more difficult for these organisms to build and maintain their shells, potentially leading to population decline and ecosystem disruption.
Practical Applications and Further Exploration
The chemistry of carbonic acid and its related ions has far-reaching implications across various scientific disciplines:
- Medicine: Understanding the bicarbonate buffer system is crucial for diagnosing and treating acid-base imbalances in patients.
- Environmental Science: Studying ocean acidification and its effects on marine ecosystems is crucial for developing strategies for mitigating climate change.
- Geochemistry: The carbonic acid system plays a significant role in weathering processes and the formation of carbonate rocks.
- Food Science: Carbonation in beverages involves the dissolution of CO₂ in water, creating carbonic acid which contributes to the fizzy sensation.
Further exploration of this topic could delve into the kinetics of the carbonic acid reactions, the influence of temperature and pressure on the equilibrium, and the detailed mechanisms of pH regulation in biological systems.
Frequently Asked Questions (FAQ)
Q1: Is carbonic acid a strong or weak acid?
A1: Carbonic acid is a weak acid. This means it only partially dissociates in water, releasing relatively few H⁺ ions compared to strong acids like hydrochloric acid (HCl).
Q2: What is the role of bicarbonate ions (HCO₃⁻)?
A2: Bicarbonate ions act as a buffer, helping to maintain a relatively stable pH in solutions. They can react with both H⁺ and OH⁻ ions, preventing large fluctuations in pH.
Q3: How does ocean acidification affect marine life?
A3: Ocean acidification, caused by increased CO₂ dissolving in seawater, lowers the pH of the ocean. This makes it harder for marine organisms like corals and shellfish to build and maintain their calcium carbonate shells and skeletons, threatening their survival.
Q4: How is the bicarbonate buffer system regulated in the human body?
A4: The bicarbonate buffer system in the human body is regulated by the lungs (controlling CO₂ levels) and the kidneys (controlling H⁺ and HCO₃⁻ excretion).
Q5: What is the difference between CO₂(aq) and H₂CO₃(aq)?
A5: CO₂(aq) represents carbon dioxide dissolved in water, while H₂CO₃(aq) represents carbonic acid, formed from the reaction of dissolved CO₂ with water. However, it's crucial to remember that only a small fraction of dissolved CO₂ actually converts to H₂CO₃. Most remains as dissolved CO₂.
Conclusion: A Vital Interplay of Chemical Species
The interrelationships between H₂O, CO₂, H₂CO₃, H⁺, and HCO₃⁻ are fundamental to understanding a broad range of natural processes and human impacts on the environment. From the intricate mechanisms maintaining pH balance in living organisms to the large-scale effects of ocean acidification, this seemingly simple chemistry holds profound implications. Further research and understanding of these interactions are critical for addressing global environmental challenges and advancing scientific knowledge in various fields. The complex interplay of these chemical species continues to fascinate scientists and highlights the interconnectedness of Earth's systems.
Latest Posts
Latest Posts
-
Graph Of 1 X 1
Sep 16, 2025
-
Covalency Of Nitrogen In Hno3
Sep 16, 2025
-
Ko2 Oxidation Number Of K
Sep 16, 2025
-
How To Find Your Phenotype
Sep 16, 2025
-
Enthalpy Of Formation Of Hydrogen
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about H2o Co2 H2co3 H+ Hco3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.