2 Methyl 1 3 Butadiene
thesills
Sep 18, 2025 · 7 min read
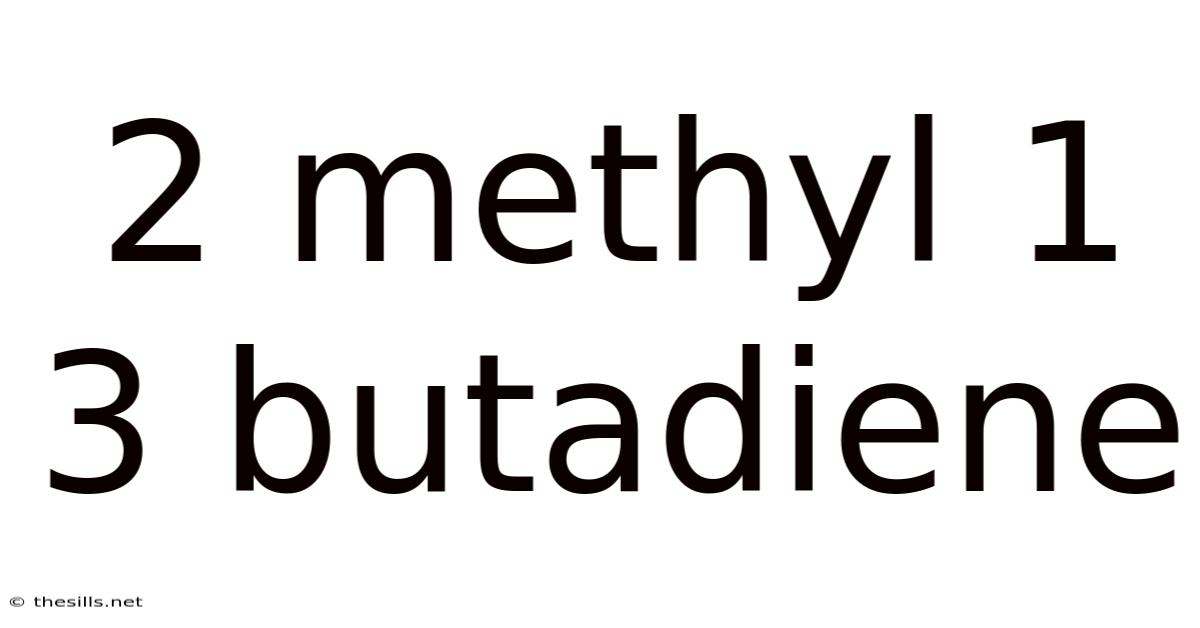
Table of Contents
Diving Deep into 2-Methyl-1,3-Butadiene: Structure, Properties, Reactions, and Applications of Isoprene
2-Methyl-1,3-butadiene, more commonly known as isoprene, is a crucial hydrocarbon with a wide range of applications. This article delves into the fascinating world of isoprene, exploring its chemical structure, physical and chemical properties, significant reactions, and diverse applications in various industries. Understanding isoprene is key to appreciating its role in everything from the creation of synthetic rubber to its natural presence in biological systems.
Introduction: Unveiling the Building Block of Life and Rubber
Isoprene, with its molecular formula C₅H₈, is a colorless, volatile liquid with a characteristic pungent odor. Its structure features a conjugated diene system, meaning it possesses two carbon-carbon double bonds separated by a single bond. This specific arrangement is crucial for its reactivity and ability to undergo polymerization. While synthetically produced, isoprene is also naturally occurring, acting as a fundamental building block in the biosynthesis of terpenoids, a vast class of organic compounds found in numerous plants, animals, and microorganisms. This makes isoprene study highly relevant across multiple scientific disciplines, from organic chemistry to biochemistry and materials science. The importance of isoprene is further emphasized by its use as a primary monomer in the production of synthetic rubber, a material that revolutionized various industries. This article will explore all of these aspects in detail, guiding you through the multifaceted nature of this remarkable molecule.
Understanding the Structure of Isoprene: A Conjugated Di-ene
The chemical structure of isoprene is characterized by its conjugated diene system. This means the two double bonds are separated by a single bond, leading to resonance stabilization and influencing its reactivity. The molecule can be represented by several structural formulas, including:
- Skeletal Formula: CH₂=C(CH₃)-CH=CH₂
- Condensed Formula: CH₂=C(CH₃)CH=CH₂
- Lewis Structure: A structure showing all atoms and bonds, highlighting the double bonds and the methyl group attached to the second carbon atom.
This conjugated structure is crucial to isoprene's ability to undergo polymerization, a key process in the production of synthetic rubbers like polyisoprene. The presence of the methyl group (CH₃) adds steric bulk, influencing the polymer's properties and the overall characteristics of the resulting material.
Physical and Chemical Properties: A Closer Look
Isoprene exhibits several key physical and chemical properties:
- Appearance: Colorless liquid
- Odor: Pungent, characteristic
- Boiling Point: Approximately 34°C (93°F) – highly volatile.
- Melting Point: Approximately -146°C (-231°F)
- Density: Lower than water
- Solubility: Slightly soluble in water, but readily soluble in organic solvents.
- Reactivity: Highly reactive due to the conjugated diene system, readily undergoing addition reactions like polymerization and Diels-Alder reactions.
The volatility of isoprene necessitates careful handling and storage to prevent evaporation and potential fire hazards. Its solubility characteristics determine its applications and processing methods. The high reactivity is the cornerstone of its use in various chemical synthesis and polymerization processes.
Key Reactions of Isoprene: Polymerization and Beyond
Isoprene's chemical reactivity, primarily stemming from its conjugated diene system, allows it to participate in various reactions:
-
Polymerization: This is arguably the most important reaction of isoprene. Polymerization is the process where many isoprene molecules link together to form long chains called polyisoprene. This process can yield both cis-polyisoprene (natural rubber) and trans-polyisoprene (gutta-percha). The difference lies in the stereochemistry around the double bonds within the polymer chain. Cis-polyisoprene is elastic and flexible, while trans-polyisoprene is rigid and hard.
-
Addition Reactions: Isoprene readily undergoes addition reactions, where molecules add across the double bonds. This is crucial in various chemical syntheses. For example, halogenation (addition of halogens like chlorine or bromine) or hydrogenation (addition of hydrogen) are possible.
-
Diels-Alder Reactions: As a conjugated diene, isoprene is an excellent diene in Diels-Alder reactions. These are [4+2] cycloaddition reactions where isoprene reacts with a dienophile (a molecule containing a multiple bond) to form a cyclic compound. This reaction is widely used in organic synthesis to construct complex ring systems.
-
Oxidation Reactions: Isoprene can undergo oxidation reactions, leading to the formation of various oxygenated derivatives. This can be controlled to create specific compounds or used in degradation studies.
Isoprene's Applications: A Versatile Molecule
The applications of isoprene are vast and span multiple industries:
-
Synthetic Rubber Production: This is arguably the largest application of isoprene. Polyisoprene, synthesized from isoprene, serves as a crucial component in various rubber products, including tires, belts, hoses, and other elastic materials. Synthetic rubber is often preferred over natural rubber due to consistent quality and availability.
-
Production of Terpenoids: While isoprene itself isn't directly used, understanding its role in terpenoid biosynthesis is crucial. Terpenoids are a large class of naturally occurring organic compounds with diverse biological activities. Many terpenoids possess medicinal properties or act as fragrances and flavoring agents.
-
Chemical Intermediate: Isoprene acts as an important intermediate in the synthesis of other chemicals. Its reactivity and structural features make it a versatile building block for creating more complex molecules with various applications.
-
Research and Development: Isoprene plays a significant role in scientific research, particularly in the fields of polymer chemistry, organic chemistry, and biochemistry. Understanding its reactions and polymerization mechanisms is crucial for developing novel materials and understanding biological processes.
-
Fuel Additive: In some specialized applications, isoprene may be used as a component in fuel blends, contributing to its improved combustion properties. However, this application is less widespread compared to its role in rubber and chemical synthesis.
The Significance of Isoprene in Biological Systems: The Isoprene Rule
Beyond its industrial applications, isoprene holds considerable biological importance. It's a major volatile organic compound (VOC) emitted by plants, and its emission plays a role in atmospheric chemistry and climate studies. Isoprene is a crucial building block in the biosynthesis of numerous terpenoids, a diverse group of molecules that play essential roles in various biological processes. The isoprene rule states that many terpenoids are composed of isoprene units linked together in different ways. This observation highlights the central role of this molecule in creating the amazing structural diversity found within the terpenoid family.
Frequently Asked Questions (FAQ) about Isoprene
Q: Is isoprene harmful?
A: Isoprene is a volatile and flammable compound. Inhalation of high concentrations can cause respiratory irritation. Direct skin contact can cause irritation. Appropriate safety precautions, including adequate ventilation and personal protective equipment (PPE), are crucial when handling isoprene.
Q: How is isoprene produced?
A: Isoprene can be produced through both natural and synthetic methods. Natural production occurs in plants and other organisms. Synthetic production primarily involves the catalytic dimerization of propylene or the dehydration of isopentenol.
Q: What is the difference between natural rubber and synthetic rubber made from isoprene?
A: Both natural rubber and synthetic cis-polyisoprene have similar chemical structures. However, natural rubber may contain impurities and variations in its structure, while synthetic rubber offers more consistent quality and properties.
Q: Is isoprene used in the production of plastics?
A: While not a primary component in many common plastics, isoprene's derivatives and polymers can contribute to certain specialized plastic formulations. Its primary role, however, remains in rubber production.
Conclusion: Isoprene – A Molecule of Multifaceted Significance
2-Methyl-1,3-butadiene, or isoprene, is a remarkable molecule with a significant impact on various industries and biological systems. Its unique conjugated diene structure underpins its high reactivity and versatility. The ability of isoprene to undergo polymerization leads to the production of synthetic rubber, a material essential for countless applications. Furthermore, its role in the biosynthesis of terpenoids highlights its importance in the natural world. Understanding isoprene's structure, properties, reactions, and applications provides valuable insights into the fields of organic chemistry, polymer science, biochemistry, and materials science. The continued study and utilization of isoprene will undoubtedly contribute to advancements in various technological and biological fields.
Latest Posts
Latest Posts
-
Is Nh3 A Strong Base
Sep 18, 2025
-
Mark Wayne Roll Top Desk
Sep 18, 2025
-
Where Are Synaptic Knobs Located
Sep 18, 2025
-
How Does Light Typically Travel
Sep 18, 2025
-
9th Grade Labeled Animal Cell
Sep 18, 2025
Related Post
Thank you for visiting our website which covers about 2 Methyl 1 3 Butadiene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.